ASTM D5997-96(2009)
(Test Method)Standard Test Method for On-Line Monitoring of Total Carbon, Inorganic Carbon in Water by Ultraviolet, Persulfate Oxidation, and Membrane Conductivity Detection
Standard Test Method for On-Line Monitoring of Total Carbon, Inorganic Carbon in Water by Ultraviolet, Persulfate Oxidation, and Membrane Conductivity Detection
SIGNIFICANCE AND USE
This test method is useful for detecting and determining organic and inorganic carbon impurities in water from a variety of sources including industrial water, drinking water, and waste water.
Measurement of these impurities is of vital importance to the operation of various industries such as power, pharmaceutical, semiconductor, drinking water treatment, and waste treatment. Semiconductor and power applications require measurement of very low organic carbon levels (TOC 1 μg/L). Applications in pharmaceutical industries range from USP purified water (TOC 500 μg/L) to cleaning applications (500 μg/L TOC 50 000 μ g/L). Drinking waters range from 100 μg/L to 25 000 μ g/L and higher. Some of these applications may include waters with substantial ionic impurities as well as organic matter.
Measurement of inorganic carbon as well as total organic carbon is highly important to some applications, such as in the power industry.
Continuous monitoring and observation of trends in these measurements are of interest in indicating the need for equipment adjustment or correction of water purification procedures.
Refer to Annex A1 for additional information regarding the significance of this test method.
SCOPE
1.1 This test method covers the on-line determination of total carbon (TC), inorganic carbon (IC), and total organic carbon (TOC) in water in the range from 0.5 μg/L to 50 000 μg/L of carbon. Higher carbon levels may be determined by suitable on-line dilution. This test method utilizes ultraviolet-persulfate oxidation of organic carbon coupled with a CO2 selective membrane to recover the CO2 into deionized water. The change in conductivity of the deionized water is measured and related to carbon concentration in the oxidized sample using calibration data. Inorganic carbon is determined in a similar manner without the requirement for oxidation. In both cases, the sample is acidified to facilitate CO2 recovery through the membrane. The relationship between the conductivity measurement and carbon concentration can be described by a set of chemometric equations for the chemical equilibrium of CO2, HCO3 −, H +, and OH −, and the relationship between the ionic concentrations and the conductivity. The chemometric model includes the temperature dependence of the equilibrium constants and the specific conductances resulting in linear response of the method over the stated range of TOC. See Test Method D4519 for a discussion of the measurement of CO2 by conductivity.
1.2 This test method has the advantage of a very high sensitivity detector that allows very low detection levels on relatively small volumes of sample. Also, the use of two measurement channels allows determination of IC in the sample independently of organic carbon. Isolation of the conductivity detector from the sample by the CO2 selective membrane results in a very stable calibration with minimal interferences.
1.3 This test method was used successfully with reagent water spiked with sodium carbonate and various organic compounds. This test method is effective with both deionized water samples and samples of high ionic strength. It is the user's responsibility to ensure the validity of this test method for waters of untested matrices.
1.4 This test method is applicable only to carbonaceous matter in the sample that can be introduced into the reaction zone. The inlet system generally limits the maximum size of particles that can be introduced. Filtration may also be used to remove particles, however, this may result in removal of organic carbon if the particles contain organic carbon.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D5997 − 96(Reapproved 2009)
Standard Test Method for
On-Line Monitoring of Total Carbon, Inorganic Carbon in
Water by Ultraviolet, Persulfate Oxidation, and Membrane
Conductivity Detection
This standard is issued under the fixed designation D5997; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope compounds. This test method is effective with both deionized
water samples and samples of high ionic strength. It is the
1.1 This test method covers the on-line determination of
user’s responsibility to ensure the validity of this test method
total carbon (TC), inorganic carbon (IC), and total organic
for waters of untested matrices.
carbon (TOC) in water in the range from 0.5 µg/L to 50000
µg/L of carbon. Higher carbon levels may be determined by 1.4 This test method is applicable only to carbonaceous
suitable on-line dilution. This test method utilizes ultraviolet- matter in the sample that can be introduced into the reaction
persulfate oxidation of organic carbon coupled with a CO zone. The inlet system generally limits the maximum size of
selective membrane to recover the CO into deionized water. particles that can be introduced. Filtration may also be used to
The change in conductivity of the deionized water is measured remove particles, however, this may result in removal of
and related to carbon concentration in the oxidized sample organic carbon if the particles contain organic carbon.
using calibration data. Inorganic carbon is determined in a
1.5 This standard does not purport to address all of the
similar manner without the requirement for oxidation. In both
safety concerns, if any, associated with its use. It is the
cases,thesampleisacidifiedtofacilitateCO recoverythrough
responsibility of the user of this standard to establish appro-
the membrane. The relationship between the conductivity
priate safety and health practices and determine the applica-
measurement and carbon concentration can be described by a
bility of regulatory limitations prior to use.
set of chemometric equations for the chemical equilibrium of
− + −
CO ,HCO ,H ,andOH ,andtherelationshipbetweenthe 2. Referenced Documents
2 3
ionic concentrations and the conductivity. The chemometric
2.1 ASTM Standards:
model includes the temperature dependence of the equilibrium
D1129Terminology Relating to Water
constants and the specific conductances resulting in linear
D1192Guide for Equipment for Sampling Water and Steam
response of the method over the stated range ofTOC. SeeTest
in Closed Conduits (Withdrawn 2003)
MethodD4519foradiscussionofthemeasurementofCO by
D1193Specification for Reagent Water
conductivity.
D2777Practice for Determination of Precision and Bias of
1.2 This test method has the advantage of a very high
Applicable Test Methods of Committee D19 on Water
sensitivity detector that allows very low detection levels on
D3370Practices for Sampling Water from Closed Conduits
relatively small volumes of sample. Also, the use of two
D4519Test Method for On-Line Determination of Anions
measurement channels allows determination of IC in the
and Carbon Dioxide in High Purity Water by Cation
sample independently of organic carbon. Isolation of the
Exchange and Degassed Cation Conductivity
conductivity detector from the sample by the CO selective
3. Terminology
membrane results in a very stable calibration with minimal
interferences.
3.1 Definitions:
3.1.1 For definitions of terms used in this test method, refer
1.3 This test method was used successfully with reagent
to Terminology D1129.
water spiked with sodium carbonate and various organic
3.2 Definitions of Terms Specific to This Standard:
This test method is under the jurisdiction ofASTM Committee D19 on Water
and is the direct responsibility of Subcommittee D19.03 on Sampling Water and For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Water-Formed Deposits,Analysis of Water for Power Generation and Process Use, contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
On-Line Water Analysis, and Surveillance of Water. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Oct. 1, 2009. Published November 2009. Originally the ASTM website.
approved in 1996. Last previous edition approved in 2000 as D5997–96 (2005). The last approved version of this historical standard is referenced on
DOI: 10.1520/D5997-96R09. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5997 − 96 (2009)
3.2.1 inorganic carbon (IC), n—carbon in the form of 5.5 Refer to AnnexA1 for additional information regarding
carbon dioxide, carbonate ion, or bicarbonate ion. the significance of this test method.
3.2.2 refractory material, n—that which cannot be oxidized
6. Interferences and Limitations
completely under the test method conditions.
6.1 The oxidation of dissolved carbon to CO is brought
3.2.3 total carbon (TC), n—the sum of IC and TOC.
about at relatively low temperatures by the chemical action of
3.2.4 total organic carbon (TOC), n—carbon in the form of
reactivespeciesproducedbyUV-irradiatedpersulfateions.Not
organic compounds.
all suspended or refractory material may be oxidized under
these conditions; analysts should take steps to determine what
4. Summary of Test Method
recovery is being obtained. This may be done by several
methods: (1) by rerunning the sample under more vigorous
4.1 Fundamentals—Carbon can occur in water as inorganic
reaction conditions; (2) by analyzing the sample by an alter-
and organic compounds.This test method can be used to make
native method known to result in full recovery; or (3)by
independent measurements of IC and TC and can also deter-
spiking samples with known refractories and determining
mine TOC as the difference between TC and IC. If IC is high
recovery.
relative to TOC, it is desirable to use a vacuum degassing unit
to reduce the IC concentration to obtain meaningful TOC
6.2 Interferences have been investigated and found to be
values by difference.
minimal under most conditions. Chloride ions above 250000
µg/Lmaycauselowresults.Followthemanufacturer’sinstruc-
4.2 The basic steps of this test method are:
tions for dealing with high-chloride interference. Other inter-
4.2.1 Conversion of remaining IC to CO by action of acid,
ferenceshavebeeninvestigatedandfoundtobeminimalunder
4.2.2 Removal of IC, if desired, by vacuum degassing,
most conditions. The membrane is hydrophobic in nature and
4.2.3 Split of flow into two streams to provide for separate
passes only gaseous materials. Potential interferences are
IC and TC measurements,
nitrite, sulfide, and high levels of hypochlorite or iodine. Refer
4.2.4 Oxidation of TC to CO by action of acid-persulfate
to Annex A1 for more information.
aided by ultraviolet (UV) radiation in the TC channel,
4.2.5 Detection of CO by passing each liquid stream over
2 6.3 Note that error will be introduced when the method of
membranes that allow the specific passage of CO to high-
difference is used to derive a relatively small level from two
purity water where change in conductivity is measured, and
large levels. For example, a water high in IC and low in TOC
4.2.6 Conversion of the conductivity detector signal to a
will give a less precise TOC value as (TC-IC) than by direct
display of carbon concentration in parts per million
measurement. In this case the vacuum degassing unit on the
(ppm=mg⁄L) or parts per billion (ppb=µg⁄L). The IC chan-
instrument should be used to reduce the concentration of IC
nel reading is subtracted from the TC channel reading to give
prior to measurement, or another method of inorganic carbon
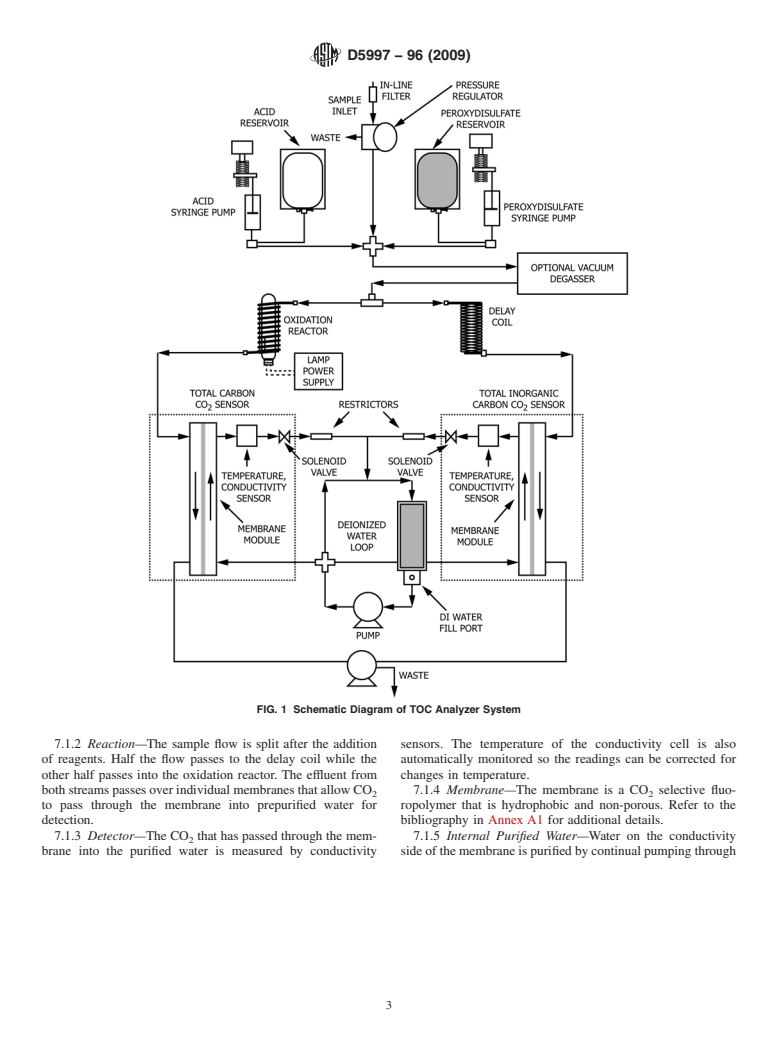
aTOCreading.AdiagramofsuitableapparatusisgiveninFig.
removal should be employed.
1.
6.4 Use of the vacuum degassing unit or sparging the
samplerenderstheICreadingmeaninglessandmaycauseloss
5. Significance and Use
of volatile organic compounds, thus yielding a value lower
5.1 This test method is useful for detecting and determining
thanthetrueTOClevel.AtlowTOClevels,thedegassingunit
organicandinorganiccarbonimpuritiesinwaterfromavariety
mayintroduceameasurableTOCandICbackground.Theuser
ofsourcesincludingindustrialwater,drinkingwater,andwaste
should characterize the background and performance of the
water.
degassing module for their applications. Table 1 provides
typical IC removal performance and background levels of the
5.2 Measurement of these impurities is of vital importance
vacuum degassing unit.
to the operation of various industries such as power,
pharmaceutical, semiconductor, drinking water treatment, and
7. Apparatus
waste treatment. Semiconductor and power applications re-
7.1 Apparatus for Carbon Determination—Atypical instru-
quire measurement of very low organic carbon levels (TOC <
ment consists of reagent and sample introduction mechanism,
1 µg/L). Applications in pharmaceutical industries range from
reaction vessel, detector, control system, and a display. Fig. 1
USPpurified water (TOC < 500 µg/L) to cleaning applications
shows a diagram of such an arrangement.
(500µg/L
7.1.1 Vacuum degassing requires the manufacturer’s
< 100 µg/L to 25000 µ g/L and higher. Some of these
module, which includes a vacuum pump and a hollow fiber
applications may include waters with substantial ionic impu-
membraneassembly.Useofthisvacuumdegasserwillremove
rities as well as organic matter.
essentiallyallICaspartoftheanalysis.Themembranemodule
5.3 Measurement of inorganic carbon as well as total
consists of a tube and shell arrangement of microporous
organic carbon is highly important to some applications, such
polypropylene hollow fibers. Sample flows along the inside of
as in the power industry.
the fibers while air is passed on the shell side, counterflow to
5.4 Continuous monitoring and observation of trends in thesampleflow.Theshellsidepressureisreducedbymeansof
these measurements are of interest in indicating the need for avacuumpumpontheairoutlet.Thesampleisacidifiedbefore
equipment adjustment or correction of water purification pro- introduction into the degasser to facilitate CO transport
cedures. through the hollow fibers.
D5997 − 96 (2009)
FIG. 1 Schematic Diagram of TOC Analyzer System
7.1.2 Reaction—The sample flow is split after the addition sensors. The temperature of the conductivity cell is also
of reagents. Half the flow passes to the delay coil while the automatically monitored so the readings can be corrected for
other half passes into the oxidation reactor. The effluent from changes in temperature.
bothstreamspassesoverindividualmembranesthatallowCO 7.1.4 Membrane—The membrane is a CO selective fluo-
2 2
to pass through the membrane into prepurified water for ropolymer that is hydrophobic and non-porous. Refer to the
detection. bibliography in Annex A1 for additional details.
7.1.3 Detector—The CO that has passed through the mem- 7.1.5 Internal Purified Water—Water on the conductivity
brane into the purified water is measured by conductivity sideofthemembraneispurifiedbycontinualpumpingthrough
D5997 − 96 (2009)
TABLE 1 Blank Contribution and IC Removal Efficiency of
solutions. Protect CO -free water from atmospheric contami-
Vacuum Degassing Unit
nation. Glass containers are required for storage of water and
TOC Background, IC Background, IC Level with
standard solutions.
Unit No.
A A
µg/L µg/L 25 000 µg/L Input
1 3.2 8.2 55
8.3 Acid Reagent (6 M)—Prepare acid solution to a concen-
2 3.2 22 61
tration of 6 M and verify that it contains less than 600 µg/L
3 2.4 8.0 105
organic carbon contamination. Since halogens are potential
4 4.2 13 89
5 2.8 13 30
interferences,useonlysulfuricorphosphoricacidforreagents.
6 3.0 8.0 70
Preparesulfuricacidbydiluting336mLof95%reagent(spgr
7 4.8 8.9 67
8 4.7 8.3 63 1.84) to 1 L with reagent water. Prepare phosphoric acid by
94.6 11 62
diluting410mLof85%reagent(spgr1.69)to1Lwithwater.
10 4.7 2.9 72
Certification of reagent assay should be available. Reagents in
A
Values are the difference between, before, and after addition of the degasser to
prepackagedcontainersfromtheinstrumentmanufacturerhave
a high-purity (<5 µg/L) water stream.
been found to be acceptable.
8.4 Persulfate Reagent (15 % w/v)—Prepare ammonium
persulfatetoaconcentrationof15%w/vbydissolving15gof
a mixed bed ion exchange resin as shown in Fig. 1. On power
ammonium peroxydisulfate in water and diluting to 100 mL.
up, the instrument automatically delays for a period of at least
Verify that it contains less than 2000 µg/L organic carbon
5 min to allow the water in the internal loop to be fully
contamination. Certification of reagent assay should be avail-
deionized. The mixed bed ion exchange resin has an expected
able. Reagents in prepackaged containers from the instrument
life of several years. See 14.3 for details on monitoring the
manufacturer have been found to be acceptable.
resin.
7.1.6 Presentation of Results—The conductivity detector 8.5 Organic Carbon Solution Standard (2000 mg/L)—
outputisrelatedtostoredcalibrationdataandthendisplayedas Choose a water-soluble, stable reagent grade compound such
parts per million (ppm=mg⁄L of carbon) or parts per billion as benzoic acid or anhydrous potassium hydrogen phthalate
(ppb=µg⁄Lof carbon). Values are given for TC, IC, and TOC (KHP, KHC H O ). Calculate the weight of compound re-
8 4 4
by difference. Data can be maintained on internal nonvolatile
quired to make 1 L of organic carbon standard solution; for
RAM, printer tape, or computer storage.
example, KHC H O =0.471 g of carbon per gram, so 1 L of
8 4 4
2 g/L of standard requires 2/0.471 or 4.25 g of KHP. Dissolve
8. Reagents and Materials
the required amount of standard in some CO -free water in a
8.1 Purity of Reagents—Use reagent grade chemicals in all 1-L volumetric flask, add 1 mL of concentrated H SO (sp gr
2 4
tests.Unlessotherwiseindicated,itisintendedthatallreagents 1.84), and dilute to volume. Dilutions of this stock solution
conform to the specifications of the Committee on Analytical containing 2 mg/L are to be used to calibrate and test
Reagents of the American Chemical Society, where such performance of the carbon analyzer.
specifications are available. Other grades may be used, pro-
8.6 Inorganic Carbon Solution Standard (2000 mg/L)—
videditisfirstascertainedthatthereagentisofsufficientpurity
Choos
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.