ASTM C186-98
(Test Method)Standard Test Method for Heat of Hydration of Hydraulic Cement
Standard Test Method for Heat of Hydration of Hydraulic Cement
SCOPE
1.1 This test method covers the determination of the heat of hydration of a hydraulic cement by measuring the heat of solution of the dry cement and the heat of solution of a separate portion of the cement that has been partially hydrated for 7 and for 28 days, the difference between these values being the heat of hydration for the respective hydrating period.
1.2 The results of this test method may be inaccurate if some of the components of the hydraulic cement are insoluble in the nitric acid/hydrofluoric acid solution.
1.3 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.4 Values in SI units shall be obtained by measurement in SI units or by appropriate conversion, using the Rules for Conversion and Rounding given in Practice E 380, or measurements made in other units.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability or regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:C186–98
Standard Test Method for
Heat of Hydration of Hydraulic Cement
This standard is issued under the fixed designation C 186; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Significance and Use
1.1 This test method covers the determination of the heat of 3.1 The purpose of this test is to determine if the hydraulic
hydration of a hydraulic cement by measuring the heat of cement under test meets the heat of hydration requirement of
solution of the dry cement and the heat of solution of a separate the applicable hydraulic cement specification.
portion of the cement that has been partially hydrated for 7 and 3.2 This test may also be used for research purposes when it
for 28 days, the difference between these values being the heat is desired to determine the heat of hydration of hydraulic
of hydration for the respective hydrating period. cement at any age.
1.2 The results of this test method may be inaccurate if
NOTE 1—When tests are performed for research purposes, useful
some of the components of the hydraulic cement are insoluble
additional information can be obtained by determining fineness, chemical
in the nitric acid/hydrofluoric acid solution.
and compound compositions.
1.3 The values stated in SI units are to be regarded as the
3.3 Determination of the heat of hydration of hydraulic
standard. The values given in parentheses are for information
cements provides information that is helpful for calculating
only.
temperature rise in mass concrete.
1.4 Values in SI units shall be obtained by measurement in
SI units or by appropriate conversion, using the Rules for
4. Apparatus
Conversion and Rounding given in Standard IEEE/ASTM SI
4.1 Calorimetric Apparatus:
10, or measurements made in other units.
4.1.1 Calorimeter—The calorimeter, such as that illustrated
1.5 This standard does not purport to address all of the
inFig.1shallconsistofa0.5-L(1-pt),wide-mouthvacuumjar,
safety concerns, if any, associated with its use. It is the
withcorkstopper,orothersuitablenon-reactivestopperheldin
responsibility of the user of this standard to establish appro-
asuitablyinsulatedcontainer(see4.1.2)tokeepthevacuumjar
priate safety and health practices and determine the applica-
in position and to protect the jar from undue temperature
bility or regulatory limitations prior to use.
fluctuations. The vacuum jar shall be coated on the interior
with a material resistant to hydrofluoric acid, such as a baked
2. Referenced Documents
phenolic resin, a baked vinyl chloride acetate resin, or bees-
2.1 ASTM Standards:
wax. The acid-resistant coating shall be intact and free of
C 109 Test Method for Compressive Strength of Hydraulic
cracksatalltimes;itshallbeexaminedfrequentlyandrenewed
Cement Mortars (Using 2-in. or 50-mm Cube Specimens)
whenever necessary. As another means of protecting the
C 114 Test Methods for Chemical Analysis of Hydraulic
vacuum jar, a plastic liner of suitable size may be used instead
Cement
of coating the interior of the jar.The contents of the vacuum jar
C 670 Practice for Preparing Precision and Bias Statements
shall not change more than 0.001°C/min per degree difference
for Test Methods for Construction Materials
from room temperature when filled with 425 g of the acid
C 1005 Specification forWeights andWeighing Devices for
specified in 6.2, stoppered, and allowed to stand unstirred for
Use in the Physical Testing of Hydraulic Cements
30 min. The temperature for this check shall approximate the
E 11 Specification for Wire-Cloth Sieves for Testing Pur-
starting temperatures to be used in making the determination.
poses
4.1.2 Insulated Container—The container shall have an
IEEE/ASTM SI 10 Standard for Use of the International
insulatinglayerofamaterialsuchasnon-reactivefoam,cotton,
System of Units (SI): The Modern Metric System
or fiber-glass, which shall be at least 25 mm (1 in.) in thickness
and shall encase the sides and bottom of the vacuum jar, but
shall be so arranged as to permit easy removal of the jar.
This test method is under the jurisdiction ofASTM Committee C-1 on Cement
and is the direct responsibility of Subcommittee C01.26 on Heat of Hydration. 4.1.3 Differential and Reference Thermometers—The ad-
Current edition approved Jan. 10, 1998. Published July 1998. Originally
justable differential thermometer shall be of the Beckmann-
published as C 186 – 44 T. Last previous edition C 186 – 97.
type, graduated at least to 0.01°C, and shall have a range of
Annual Book of ASTM Standards, Vol 04.01.
approximately 6°C. The thermometer shall be so adjusted that
Annual Book of ASTM Standards, Vol 04.02.
Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
C186–98
FIG. 1 Calorimeter
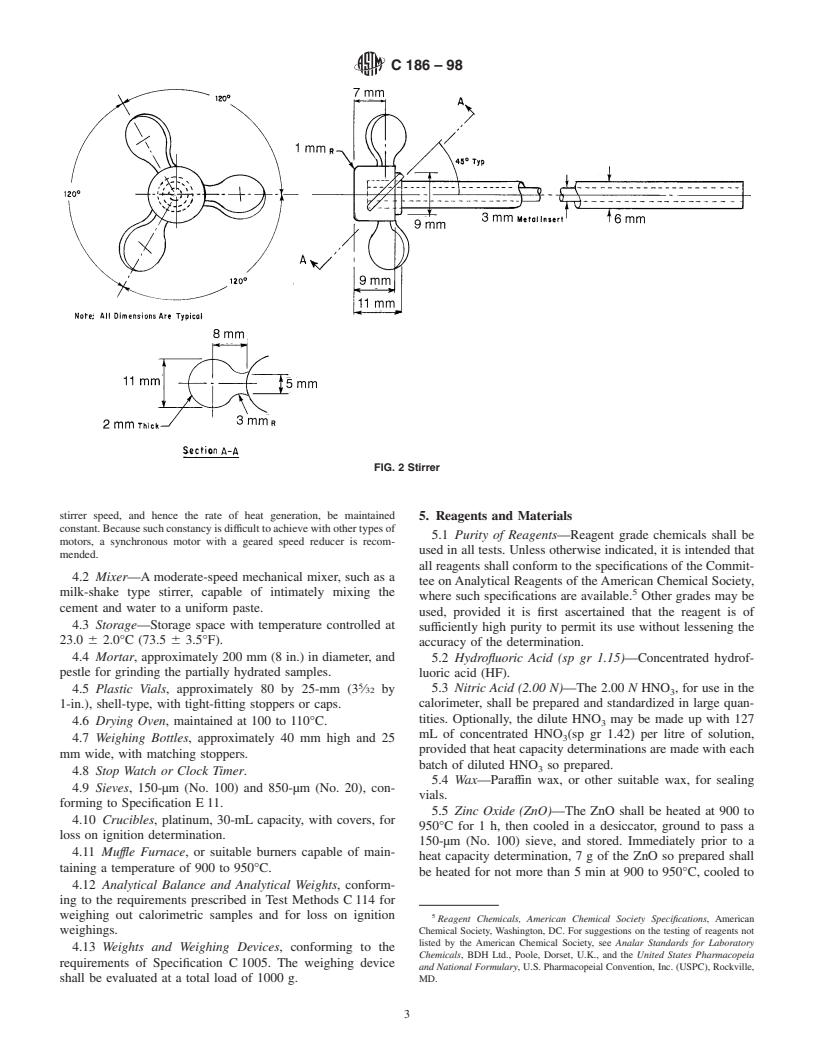
the upper limit of the scale approximates room temperature. 4.1.5 Stirring Assembly—The stirrer shall be a three-bladed
The portion of the thermometer that will rest inside the polyethylene propeller having the dimensions shown in Fig. 2,
calorimeter shall be protected with a coating resistant to
and shall extend as closely as possible to the bottom of the
hydrofluoric acid (see 4.1.1). The thermometer shall be
calorimeter. The motor shall be of the constant-speed type, at
equipped with a suitable reading lens. The Beckmann ther-
least 37 W ( ⁄20 hp), and shall be equipped with a geared speed
mometerzeromustbedeterminedbyimmersioninaliquidand
reducer so that one speed, in the range of 350 to 700 r/min, can
comparison with the reference thermometer described. An
be maintained constant.
accurate reference thermometer of the appropriate range and
NOTE 2—The stirrer shown in Fig. 2 may be readily made from a
having 0.1°C divisions shall be placed in the proximity of the
commercially available three-bladed polyethylene propeller having a
calorimetric apparatus and shall be used for room temperature
3 1
propeller diameter of 34 mm (1 ⁄8 in.), shaft diameter of 6 mm ( ⁄4 in.), and
readings and for establishing the Beckmann thermometer zero.
a shaft length of approximately 455 mm (18 in.). The function of the
4.1.4 Funnel—The funnel through which the sample is
stirrer is two-fold: to maintain uniform temperature throughout the liquid
introduced into the calorimeter shall be of glass or plastic and
andtosupplysufficientagitationtokeepthesolidinsuspensionintheacid
shall have a stem approximately 75 mm (3 in.) in length and
mixture. Since a stirrer capable of keeping the solid in suspension
with an inside diameter of not less than 6 mm ( ⁄4 in.). generates considerable heat in the calorimeter, it is important that the
C186–98
FIG. 2 Stirrer
stirrer speed, and hence the rate of heat generation, be maintained
5. Reagents and Materials
constant.Becausesuchconstancyisdifficulttoachievewithother types of
5.1 Purity of Reagents—Reagent grade chemicals shall be
motors, a synchronous motor with a geared speed reducer is recom-
used in all tests. Unless otherwise indicated, it is intended that
mended.
all reagents shall conform to the specifications of the Commit-
4.2 Mixer—A moderate-speed mechanical mixer, such as a
tee on Analytical Reagents of the American Chemical Society,
milk-shake type stirrer, capable of intimately mixing the
where such specifications are available. Other grades may be
cement and water to a uniform paste.
used, provided it is first ascertained that the reagent is of
4.3 Storage—Storage space with temperature controlled at
sufficiently high purity to permit its use without lessening the
23.0 6 2.0°C (73.5 6 3.5°F).
accuracy of the determination.
4.4 Mortar, approximately 200 mm (8 in.) in diameter, and
5.2 Hydrofluoric Acid (sp gr 1.15)—Concentrated hydrof-
pestle for grinding the partially hydrated samples. luoric acid (HF).
4.5 Plastic Vials, approximately 80 by 25-mm (3 ⁄32 by 5.3 Nitric Acid (2.00 N)—The 2.00 N HNO , for use in the
calorimeter, shall be prepared and standardized in large quan-
1-in.), shell-type, with tight-fitting stoppers or caps.
tities. Optionally, the dilute HNO may be made up with 127
4.6 Drying Oven, maintained at 100 to 110°C.
mL of concentrated HNO (sp gr 1.42) per litre of solution,
4.7 Weighing Bottles, approximately 40 mm high and 25
provided that heat capacity determinations are made with each
mm wide, with matching stoppers.
batch of diluted HNO so prepared.
4.8 Stop Watch or Clock Timer.
5.4 Wax—Paraffin wax, or other suitable wax, for sealing
4.9 Sieves, 150-µm (No. 100) and 850-µm (No. 20), con-
vials.
forming to Specification E 11.
5.5 Zinc Oxide (ZnO)—The ZnO shall be heated at 900 to
4.10 Crucibles, platinum, 30-mL capacity, with covers, for
950°C for 1 h, then cooled in a desiccator, ground to pass a
loss on ignition determination.
150-µm (No. 100) sieve, and stored. Immediately prior to a
4.11 Muffle Furnace, or suitable burners capable of main-
heat capacity determination,7goftheZnOso prepared shall
taining a temperature of 900 to 950°C.
be heated for not more than 5 min at 900 to 950°C, cooled to
4.12 Analytical Balance and Analytical Weights, conform-
ing to the requirements prescribed in Test Methods C 114 for
weighing out calorimetric samples and for loss on ignition
Reagent Chemicals, American Chemical Society Specifications, American
weighings. Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory
4.13 Weights and Weighing Devices, conforming to the
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
requirements of Specification C 1005. The weighing device
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
shall be evaluated at a total load of 1000 g. MD.
C186–98
room temperature in a desiccator, and weighed accurately for
u = calorimeter temperature at the end of the rating
introduction into the calorimeter.
period.
6.5 Calculate the heat capacity of the calorimeter and
NOTE 3—The rate of solution of the ZnO varies with the preliminary
contents as follows (see Note 5):
treatment. The procedure described results in a product which dissolves at
about the same rate as the dry cement.
W[1072 1 0.4~30 2 t! 1 0.5~T 2 t!#
C 5 (2)
R
6. Determination of Heat Capacity of Apparatus
6.1 To determine the heat capacity of the system (that is, the
where:
number of joules or calories required to raise the temperature
C = heat capacity, kJ/°C,
of the calorimeter and contents 1°C), measure the corrected
W = mass of ZnO, g,
temperature rise obtained by dissolving7gof ignited ZnO in
t = final temperature of the calorimeter, °C (u plus
the specified acid mixture (see 6.2-6.7).
temperature, °C, at which the Beckmann thermometer
6.2 Transfer approximately 400 g of the 2.00 N HNO ,
reading is zero),
which has been cooled to the temperature indicated by the
T = temperature of the ZnO (room temperature), °C, when
lower range of the Beckmann thermometer (ordinarily about 4
introduced into the calorimeter, and
to 5°C below room temperature), into the vacuum jar, add 8.0
R = corrected temperature rise, °C.
mL of HF (sp gr 1.15), weigh, and add sufficient additional
NOTE 5—The heat of solution of ZnO is 1072 kJ/kg (256.1 cal/g) at
2.00 N HNO to bring the total weight of the solution to 425.0
30°C. This value increases 0.4 kJ/kg (0.1 cal/g) for each degree decrease
g. Then, assemble the calorimeter and start the stirring motor.
in temperature below 30°C. The heat capacity of ZnO is 0.5 kJ/kg·K (0.12
Take care that the stirrer blades or shaft do not touch the
cal/g·°C). The heat required to bring the ZnO to the final temperature of
thermometer, the sides or bottom of the jar, or the cork stopper.
the calorimeter must be included in the effective heat of solution.
The lower end of the funnel stem shall extend approximately 6
6.6 If more than a trace of ZnO is found adhering to the tip
mm ( ⁄4 in.) below the lower surface of the stopper and at least
of the funnel or to the stopper when the calorimeter is opened,
12 mm ( ⁄2 in.) above the level of the liquid. The upper end of
reject the test.
the bulb of the Beckmann thermometer shall be at least 38 mm
6.7 Redetermine the heat capacity at the following times:
(1 ⁄2 in.) below the surface of the liquid. Place it at the same
6.7.1 When the Beckmann thermometer is reset,
depth in all determinations.After an initial stirring period of at
6.7.2 When a new coating is applied to thermometer, stirrer,
least 20 min to allow the temperature of the system to become
or flask,
uniform, record the temperature of the room to the nearest
6.7.3 When a new thermometer, stirrer, or flask is put in
0.1°C, the temperature of the acid to the nearest 0.001°C,
service,
record the time, and then immediately introduce the prepared
6.7.4 When a new batch of acid is used, and
ZnO through the funnel at a uniform rate (see Note 4).
6.7.5 At other times when, according to the judgment of the
CompletetheintroductionoftheZnOinnotlessthan1ormore
operator, the need is indicated.
than 2 min. Brush any ZnO clinging to the funnel stem into the
acid mixture by means of a small “camel’s-hair” brush.
7. Sampling and Test Specimens
NOTE 4—The temperature of the sample shall be identical with that of
7.1 Preparation of Cement Paste—Store the cement and the
the room when the sample is introduced into the calorimeter.
mixing water in a constant-temperature room at 23.0 6 2.0 °C
6.3 Read the temperature, to the nearest 0.001°C, at 20 min
(73.5 6 3.5°F) until the materials are at ambient temperature
and again at 40 min after beginning the introduction of the
before preparation of the paste. Mix 150 g of cement and 60
sample. The first 20-min period is the uncorrected temperature
mL of distilled water by means of a spatula, and then
rise, which covers the solution period. The second 20-min
vigorously stir the mixture with a mechanical stirrer for 5 min.
period is the rating period, and the temperature difference
Place approximately equal representative portions of the paste
between the 20 and 40-min readings is the correction to be
in four or more plastic vials, filling the vials to within about 13
added to or subtracted from the uncorrected temperature rise,
mm ( ⁄2 in.) of the top. Immediately after filling the vials, close
according to whether the calorimeter temperature rises or falls
them with tight-fitt
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.