ASTM D5464-93(2001)
(Test Method)Standard Test Methods for pH Measurement of Water of Low Conductivity
Standard Test Methods for pH Measurement of Water of Low Conductivity
SCOPE
1.1 These test methods are applicable to determine the pH of water samples with a conductivity lower than 100 µ S/cm (see Annex A1 and Table A1.1 and Table A1.2 ) over the pH range of 3 to 11 (see Fig. 1). pH measurements of water of low conductivity are problematical (see Annex A2). Specifically, these test methods avoid contamination of the sample with atmospheric gases (see Section 7) and prevent volatile components of the sample from escaping. These test methods provide for pH electrodes and apparatus that address the considerations discussed in Annex A2. These test methods also minimize problems associated with the sample's pH temperature coefficient when the operator uses these test methods to calibrate an on-line pH monitor or controller (see Appendix X1). Two test methods are given as follows:Test MethodSectionsTest Method A-Precise pH Measurement of LowConductivity Water Utilizing the Real-TimeFlowing Sample Procedure5 to 12Test Method B-pH Measurement of Low Conductivity Water Utilizing the Static Grab SampleProcedure13 to 20
1.2 Test Method A covers the precise measurement of pH in water of low conductivity utilizing a real-time, short duration, flowing sample procedure.
1.3 Test Method B covers the measurement of pH in water of low conductivity with a lower limit of 2.0 S/cm, utilizing a static grab-sample procedure where it is not practicable to take a real-time flowing sample.
Note 1--Test Method A is preferred over Test Method B whenever possible. Test Method A is not subject to the limited conductivity range, temperature interferences, potential KCl contamination, and time limitations found with Test Method B.
1.4 The values stated in SI units are to be regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D 5464–93 (Reapproved 2001)
Standard Test Methods for
pH Measurement of Water of Low Conductivity
This standard is issued under the fixed designation D 5464; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 These test methods are applicable to determine the pH
of water samples with a conductivity lower than 100 µS/cm
(see Annex A1 and Table A1.1 and Table A1.2) over the pH
rangeof3to11(seeFig.1).pHmeasurementsofwateroflow
conductivity are problematical (see Annex A2). Specifically,
these test methods avoid contamination of the sample with
atmospheric gases (see Section 7) and prevent volatile compo-
nentsofthesamplefromescaping.Thesetestmethodsprovide
forpHelectrodesandapparatusthataddresstheconsiderations
discussed in Annex A2. These test methods also minimize
problems associated with the sample’s pH temperature coeffi-
cient when the operator uses these test methods to calibrate an
on-line pH monitor or controller (seeAppendix X1). Two test
methods are given as follows:
Test Method Sections
Test Method A—Precise pH Measurement of Low 5 to 12
Conductivity Water Utilizing the Real-Time
Flowing Sample Procedure
Test Method B—pH Measurement of Low Con- 13 to 20
ductivity Water Utilizing the Static Grab Sample
Procedure
1.2 TestMethodAcoverstheprecisemeasurementofpHin
water of low conductivity utilizing a real-time, short duration,
flowing sample procedure.
1.3 Test Method B covers the measurement of pH in water
of low conductivity with a lower limit of 2.0 µS/cm, utilizing
a static grab-sample procedure where it is not practicable to
take a real-time flowing sample.
NOTE 1—Test Method A is preferred over Test Method B whenever
FIG. 1 Restrictions Imposed by the Conductivity-pH Relationship
possible. Test Method A is not subject to the limited conductivity range,
temperature interferences, potential KCl contamination, and time limita-
2. Referenced Documents
tions found with Test Method B.
2.1 ASTM Standards:
1.4 The values stated in SI units are to be regarded as
D1067 Test Methods for Acidity or Alkalinity of Water
standard.
D1129 Terminology Relating to Water
1.5 This standard does not purport to address all of the
D1193 Specification for Reagent Water
safety concerns, if any, associated with its use. It is the
D1293 Test Methods for pH of Water
responsibility of the user of this standard to establish appro-
D2777 Practice for Determination of Precision and Bias of
priate safety and health practices and determine the applica-
Applicable Methods of Committee D-19 on Water
bility of regulatory limitations prior to use.
D4453 Practice for Handling Ultra-Pure Water Samples
D5128 TestMethodforOn-LinepHMeasurementofWater
of Low Conductivity
These test methods are under the jurisdiction of ASTM Committee D19 on
Water and are the direct responsibility of Subcommittee D19.03 on Sampling of
Water and Water-Formed Deposits, Surveillance o f Water, and Flow Measurement
of Water.
Current edition approved Sept. 15, 1993. Published December 1993.
Annual Book of ASTM Standards, Vol 11.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 5464–93 (2001)
3. Terminology
3.1 Definitions—For definitions of terms used in these test
methods, refer to Terminology D1129.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 liquid junction potential—a dc potential which ap-
pears at the point of contact between the reference electrode’s
salt bridge and the sample solution. Ideally this potential is
near zero, and is stable. However, in low conductivity water it
becomes larger by an unknown amount, and is a zero offset
(1).
4. Reagents
4.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
all reagents conform to the specifications of the Committee on
Analytical Reagents of theAmerican Chemical Society where
such specifications are available. Other grades may be used,
provided it is first ascertained that the reagent is of sufficiently
high purity to permit its use without lessening the accuracy of
the determination.
4.2 Purity of Water— Unless otherwise indicated, refer-
ences to water shall be understood to mean reagent water as
defined by Type II of Specification D1193.
4.3 Commercial Buffer Solutions—Commercially available
prepared buffers traceable to NIST standards should be ad-
equate to perform the calibration procedures in 10.1-10.4.
ThesecommercialbuffersolutionsusuallyhavepHvaluesnear
4.01, 6.86, and 10.01 pH at 25°C. The exact pH of the buffer
will change with temperature and this pH versus temperature
data will be provided by the purveyor of the specific buffer.
RefertoTestMethodsD1293,MethodAforthepreparationof
reference buffer solutions if desired.
4.4 Buffer A—Commercially available 7.00 pH buffer.
4.5 Buffer B—Commercially available 4.00 pH buffer.
4.6 Buffer C—Commercially available 10.00 pH buffer.
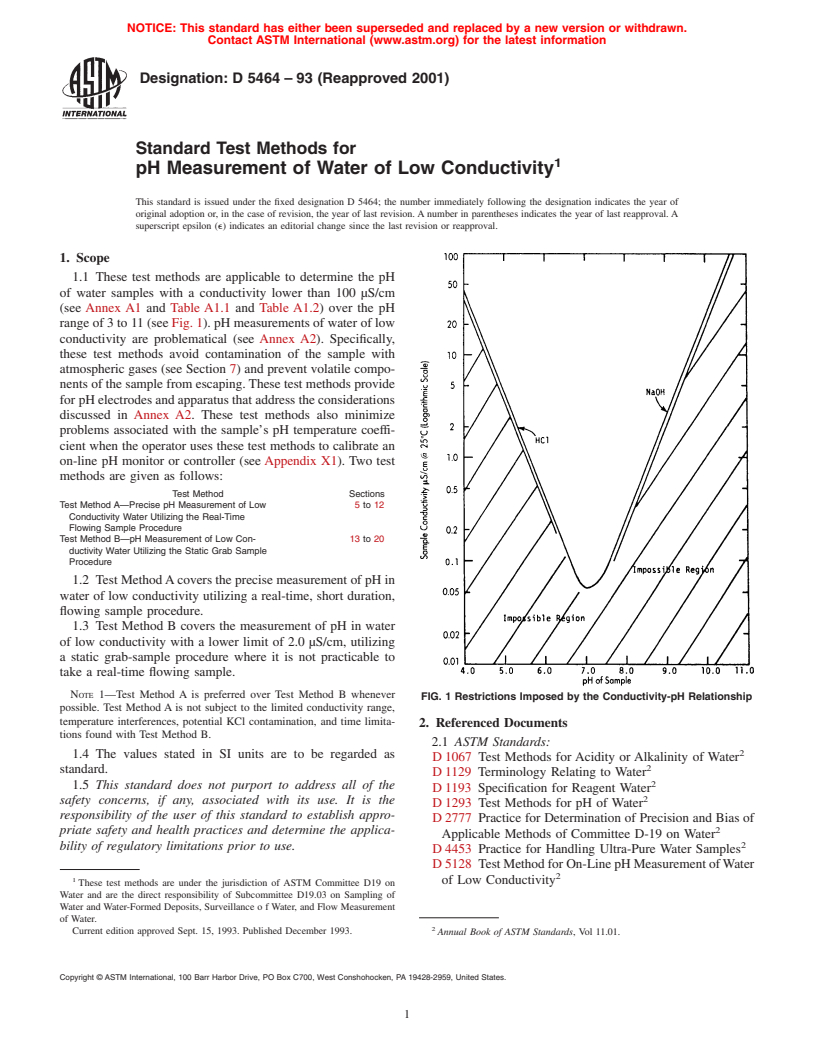
FIG. 2 Exploded View of Sample Chamber
TEST METHOD A—PRECISE pH MEASUREMENT
OF LOW CONDUCTIVITY WATER UTILIZING THE
sample chamber prevents the flowing sample from being
REAL-TIME FLOWING SAMPLE PROCEDURE
exposed to the atmosphere. This sample chamber has the pH
and reference electrode inserted into the top through gas tight
5. Summary of Test Method
fittings. The temperature compensator (if used) is inserted in a
5.1 The pH meter and associated electrodes are first stan-
like manner (see Fig. 2 and Fig. 3). The pH of the flowing
dardizedwithtwocalibrationpHbuffers.ThepHandreference
sample is measured only after the sample chamber has been
electrodesandtheautomatictemperaturecompensator(ifused)
flushed out with sample water and purged of all air.
must be removed from the sample chamber (see Fig. 2)to
5.3 pH measurement of the sample is made with a high
proceed with this calibration. The complete calibration proce-
purity water pH calibration kit comprised of a sample cham-
dure is given in Section 10 of this test method.
ber, pH and reference electrodes, and automatic temperature
5.2 Areal-time flowing grab sample is taken by means of a
compensator (if used). No other type of electrode(s) and pH
flow-through sample chamber with the inlet located at the
calibration kit have yet been validated for use with this test
bottom and the outlet located at the top of the chamber. The
method. The sample chamber should accommodate electrodes
with an outside diameter of 12 mm 6 0.20 mm (0.472 in. 6
0.008 in.). This is the standard outside diameter size for most
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
pH electrodes manufactured in the United States and Europe.
this standard.
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia Commercially available from Broadley-James Corporation, HPW pH Cal-Kit
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, Series, SantaAna, CA; Leeds & Northrup, 7082-90/7773 Series, North Wales, PA.;
MD. or equivalent.
D 5464–93 (2001)
flowing sample long enough to permit temperature equilibra-
tion before any measurements are taken.
5.6 The flow rate of the sample through the chamber must
be controlled and held constant in order to obtain repeatable
results. Each manufacturer of sample chamber must specify a
constantflowratethatisoptimumfortheirparticularapparatus.
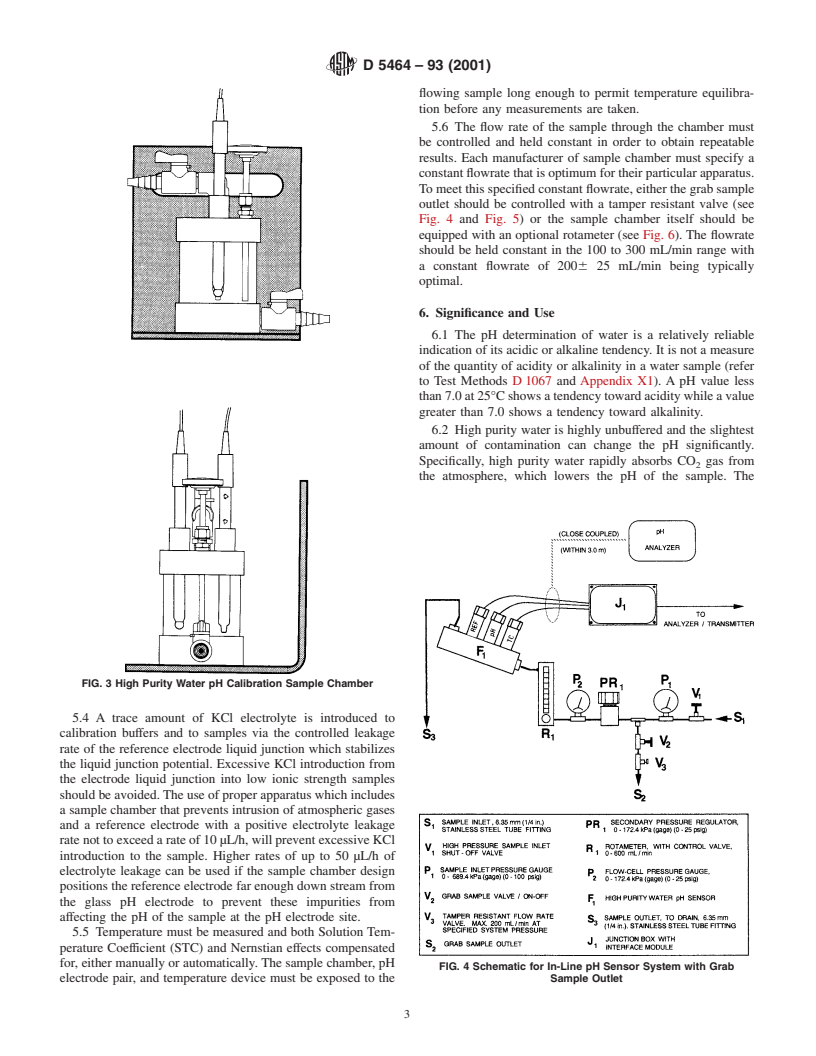
Tomeetthisspecifiedconstantflowrate,eitherthegrabsample
outlet should be controlled with a tamper resistant valve (see
Fig. 4 and Fig. 5) or the sample chamber itself should be
equipped with an optional rotameter (see Fig. 6). The flowrate
should be held constant in the 100 to 300 mL/min range with
a constant flowrate of 2006 25 mL/min being typically
optimal.
6. Significance and Use
6.1 The pH determination of water is a relatively reliable
indication of its acidic or alkaline tendency. It is not a measure
of the quantity of acidity or alkalinity in a water sample (refer
to Test Methods D1067 and Appendix X1). A pH value less
than7.0at25°Cshowsatendencytowardaciditywhileavalue
greater than 7.0 shows a tendency toward alkalinity.
6.2 High purity water is highly unbuffered and the slightest
amount of contamination can change the pH significantly.
Specifically, high purity water rapidly absorbs CO gas from
the atmosphere, which lowers the pH of the sample. The
FIG. 3 High Purity Water pH Calibration Sample Chamber
5.4 A trace amount of KCl electrolyte is introduced to
calibration buffers and to samples via the controlled leakage
rate of the reference electrode liquid junction which stabilizes
the liquid junction potential. Excessive KCl introduction from
the electrode liquid junction into low ionic strength samples
shouldbeavoided.Theuseofproperapparatuswhichincludes
a sample chamber that prevents intrusion of atmospheric gases
and a reference electrode with a positive electrolyte leakage
ratenottoexceedarateof10µL/h,willpreventexcessiveKCl
introduction to the sample. Higher rates of up to 50 µL/h of
electrolyte leakage can be used if the sample chamber design
positions the reference electrode far enough down stream from
the glass pH electrode to prevent these impurities from
affecting the pH of the sample at the pH electrode site.
5.5 Temperature must be measured and both Solution Tem-
perature Coefficient (STC) and Nernstian effects compensated
for, either manually or automatically.The sample chamber, pH
FIG. 4 Schematic for In-Line pH Sensor System with Grab
electrode pair, and temperature device must be exposed to the Sample Outlet
D 5464–93 (2001)
FIG. 5 Sample Chamber Flow Scheme
sample chamber and accompanying pH measurement tech-
nique avoid exposure of the high purity water sample to the
atmosphere.
6.3 The high purity water sample may contain volatile trace
components that will rapidly dissipate from the sample if
exposed to the atmosphere. The sample chamber used in this
test method will prevent these losses.
6.4 High purity water has a significant solution temperature
coefficient. For greatest accuracy the sample to be measured
should be at the same temperature as the sample stream. By
taking a flowing grab sample at the sample line, the operator
FIG. 6 Sample Chamber with Integral Rotameter
will use the sample water itself to bring the sample chamber
and the measurement electrodes to the sample-line tempera-
8. Apparatus
ture.
8.1 Laboratory pH Meter—See 10.1 in Test Methods
7. Interferences
D1293; or use an equivalent portable pH meter.
7.1 High purity, low conductivity samples are especially
8.2 Sample Chamber— A high purity water pH calibration
sensitive to contamination from atmospheric gases, from chamber isrequired(refertoFig.3).Thechamberenablesthe
sample containers, and from sample handling techniques and operator to measure the pH of a real-time flowing sample of
excessive KCl contamination from reference electrode or water without exposing the sample to atmospheric gases. The
samplepreparationsuchasaKCl“dosing”technique.Referto chamberisconnectedtothesamplelineviaavinyltube.Vinyl
Practice D4453 and ASTM STP 823 (2) for discussions of tubing shall be a laboratory grade which will not affect
sample handling and avoidance of sample contamination. analyses made on solutions or gases which are put through it.
7.2 Specifically, high purity water will rapidly absorb CO Thesampleflowsintothechamberthroughthebottomportand
from the atmosphere and this will lower the pH of the sample. out of the chamber through the top port. The chamber has
See Appendix X4, Table X4.1, and Fig. X4.1. o-ring sealed access ports for the insertion of a pH electrode
7.3 Thetemperaturestabilityofthesampleandhowclosely and a reference electrode.Additionally, the chamber has a 316
the sample’s temperature matches the sample stream’s tem- stainless steel gland fitting for an automatic temperature
perature will have a direct effect on accuracy of the pH compensator or a direct-reading temperature measurement
determination. For a discussion of temperature effects on pH device. The chamber is portable, with an integral stand and
measurements of high purity water see Appendix X1. carrying handle (see Fig. 2 and Fig. 3).
D 5464–93 (2001)
8.3 Rotameter (optional)—The flow rate of the sample oughly rinse electrode pair and glassware with water three
chamber must be controlled and stabilized in order to obtain times between each buffer calibration.
repeatable results (see Fig. 6). Some chambers are available
10.4 Obtain calibration precision of the pH electrode pair
with an integral rotameter.
and the pH meter by repeating the two-point calibration
8.4 pH Glass Electrode—The pH response of the glass
described in 10.3, making any necessary readjustments to the
electrode shall conform to the requirements set forth in 12.1
pHmeter.Iftheelectrodeslope(efficiency)islessthan94%or
through 12.5 of Test Methods D1293. New glass electrodes
greater than 101%, refer to manufacturer’s instructions for
and those that have been stored dry shall be conditioned and
repairorreplacementofelectrodes.Thoroughlyrinseelectrode
maintained as recommended by the manufacturer.
pair and glassware with water three times between each buffer
8.5 Reference Electrode—Double junction design, having a
calibration.
flowing junction with a positive electrolyte leakage rate not to
NOTE 2—The pH electrodes in use may pass the above calibration
exceed a rate of 10 µL/h (see 5.4). Prepare and maintain the
procedures(see10.1-10.4),butcautionshouldbetaken.pHelectrodesthat
reference electrode according to the manufacturer’s instruc-
are not specifically designed for use in high purity water may develop
tions. When using a sample chamber designed for high
problems with liquid junction potential during actual test measurements.
electrolyte leakage rates, a reference electrode with a maxi-
10.5 Determine the frequency of the two-point calibration
mum rate of 50 µL/h may be used.
oftheelectrodepairandthepHmeterbasedonusage.Perform
8.6 Temperature Compensator—See paragraph 10.4 in Test
calibration at least daily when pure water sample testing is
Methods D1293. The automatic temperature compensator
performed daily. For le
...






Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.