ASTM F2722-21
(Practice)Standard Practice for Evaluating Mobile Bearing Knee Tibial Baseplate Rotational Stops
Standard Practice for Evaluating Mobile Bearing Knee Tibial Baseplate Rotational Stops
SIGNIFICANCE AND USE
4.1 Fundamental aspects of this practice include the use of dynamic rotational force and motion representative of the human knee joint during an activity of daily living (deep flexion) and the effect of these forces and motions on the design features which stop or limit rotation in a mobile bearing knee design.
4.2 This test is required if rotational stops are designed to limit motion to ±20° or less; or there are other resistances to rotational motion with this ±20° range. In some instances, the rotational displacement could occur in both the inferior and superior interfaces.
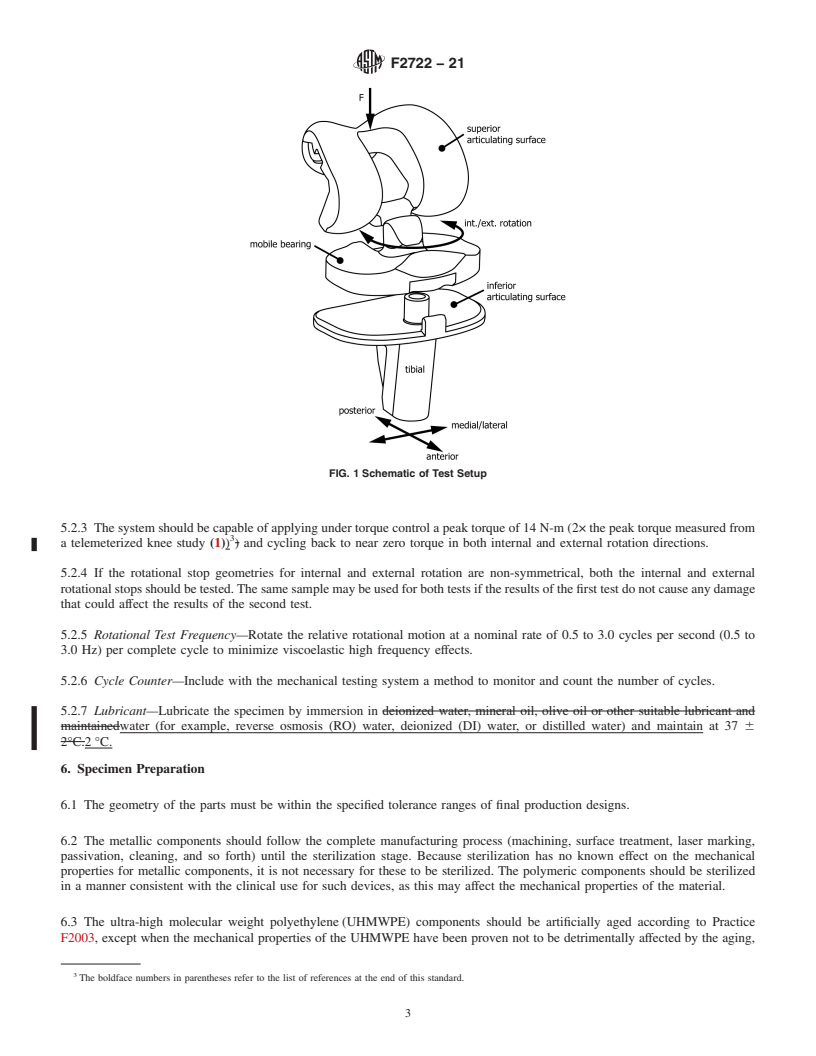
FIG. 1 Schematic of Test Setup
SCOPE
1.1 This practice covers a laboratory-based in-vitro method for evaluating the mechanical performance of materials and devices being considered for replacement of the tibio-femoral joint in human knee joint replacement prostheses in mobile bearing knee systems.
1.2 Mobile bearing knee systems permit internal/external rotation to take place on one or both articulating surfaces. Some designs place physical limits or stops to the amount of rotation. Other designs may have increases of a resistance force with increases in rotation.
1.3 Although the methodology describes attempts to identify physiologically relevant motions and force conditions, the interpretation of results is limited to an in-vitro comparison between mobile bearing knee designs and their ability to maintain the integrity of the rotational stop feature and tibial bearing component under the stated test conditions.
1.4 This practice is only applicable to mobile knee tibial systems with a rotational stop.
1.5 The values stated in SI units are regarded as standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2722 − 21
Standard Practice for
Evaluating Mobile Bearing Knee Tibial Baseplate Rotational
1
Stops
This standard is issued under the fixed designation F2722; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
2.1 ASTM Standards:
1.1 This practice covers a laboratory-based in-vitro method
F2003Practice for Accelerated Aging of Ultra-High Mo-
for evaluating the mechanical performance of materials and
lecular Weight Polyethylene after Gamma Irradiation in
devices being considered for replacement of the tibio-femoral
Air
joint in human knee joint replacement prostheses in mobile
F2083Specification for Knee Replacement Prosthesis
bearing knee systems.
1.2 Mobile bearing knee systems permit internal/external 3. Terminology
rotation to take place on one or both articulating surfaces.
3.1 Definitions:
Some designs place physical limits or stops to the amount of
3.1.1 bearing axis—thelineconnectingthelowestpointson
rotation.Otherdesignsmayhaveincreasesofaresistanceforce
both the lateral and medial condyles of the superior surface of
with increases in rotation.
the mobile bearing.
3.1.2 inferior articulating interfaces—any interface in
1.3 Although the methodology describes attempts to iden-
which relative motion occurs between the underside of the
tify physiologically relevant motions and force conditions, the
mobile bearing component and the tibial tray.
interpretation of results is limited to an in-vitro comparison
3.1.3 mobile bearing—the component between fixed femo-
between mobile bearing knee designs and their ability to
ral and tibial knee components with an articulating surface on
maintain the integrity of the rotational stop feature and tibial
both the inferior and superior sides.
bearing component under the stated test conditions.
3.1.4 mobile bearing knee system—a knee prosthesis
1.4 This practice is only applicable to mobile knee tibial
system, comprised of a tibial component, a mobile bearing
systems with a rotational stop.
component that can rotate or rotate and translate relative to the
tibial component, and a femoral component.
1.5 The values stated in SI units are regarded as standard.
3.1.5 neutral point—midpoint of the bearing axis.
1.6 This standard does not purport to address all of the
3.1.6 rotational stop—a feature that prevents relative rota-
safety concerns, if any, associated with its use. It is the
tion between two articulating joint surfaces beyond a specific
responsibility of the user of this standard to establish appro-
angle of rotation or creates resistance to rotation beyond a
priate safety, health, and environmental practices and deter-
specific angel of rotation.
mine the applicability of regulatory limitations prior to use.
3.1.7 superior articulating interfaces—any interface in
1.7 This international standard was developed in accor-
whichrelativemotionoccursbetweenthetopsideofthemobile
dance with internationally recognized principles on standard-
bearing component and the femoral bearing component.
ization established in the Decision on Principles for the
Development of International Standards, Guides and Recom-
4. Significance and Use
mendations issued by the World Trade Organization Technical
4.1 Fundamental aspects of this practice include the use of
Barriers to Trade (TBT) Committee.
dynamic rotational force and motion representative of the
human knee joint during an activity of daily living (deep
1
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devices and is the direct responsibility of Subcommittee
2
F04.22 on Arthroplasty. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Feb. 15, 2021. Published February 2021. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2008. Last previous edition approved in 2015 as F2722–15. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F2722-21. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2722 − 21
flexion) and the effect of these forces and motions on the 5.2.2 The system should be capable of maintaining an axial
designfeatureswhichstoporlimitrotationinamobilebearing force of 2000 N force as illustrated in Fig. 1. (Alt
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2722 − 15 F2722 − 21
Standard Practice for
Evaluating Mobile Bearing Knee Tibial Baseplate Rotational
1
Stops
This standard is issued under the fixed designation F2722; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This practice covers a laboratory-based in vitroin-vitro method for evaluating the mechanical performance of materials and
devices being considered for replacement of the tibio-femoral joint in human knee joint replacement prostheses in mobile bearing
knee systems.
1.2 Mobile bearing knee systems permit internal external internal/external rotation to take place on one or both articulating
surfaces. Some designs place physical limits or stops to the amount of rotation. Other designs may have increases of a resistance
force with increases in rotation.
1.3 Although the methodology describes attempts to identify physiologically relevant motions and force conditions, the
interpretation of results is limited to an in vitroin-vitro comparison between mobile bearing knee designs and their ability to
maintain the integrity of the rotational stop feature and tibial bearing component under the stated test conditions.
1.4 This practice is only applicable to mobile knee tibial systems with a rotational stop.
1.5 The values stated in SI units are regarded as standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
F2083 Specification for Knee Replacement Prosthesis
F2003 Practice for Accelerated Aging of Ultra-High Molecular Weight Polyethylene after Gamma Irradiation in Air
F2083 Specification for Knee Replacement Prosthesis
1
This practice is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee F04.22
on Arthroplasty.
Current edition approved Jan. 15, 2015Feb. 15, 2021. Published February 2015.February 2021. Originally approved in 2008. Last previous edition approved in 20082015
as F2722F2722 – 15.-08. DOI: 10.1520/F2722-15.10.1520/F2722-21.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2722 − 21
3. Terminology
3.1 Definitions:
3.1.1 bearing axis—the line connecting the lowest points on both the lateral and medial condyles of the superior surface of the
mobile bearing.
3.1.2 inferior articulating interfaces—any interface in which relative motion occurs between the underside of the mobile bearing
component and the tibial tray.
3.1.3 mobile bearing—the component between fixed femoral and tibial knee components with an articulating surface on both the
inferior and superior sides.
3.1.4 mobile bearing knee system—a knee prosthesis system, comprised of a tibial component, a mobile bearing component that
can rotate or rotate and translate relative to the tibial component, and a femoral component.
3.1.5 neutral point—midpoint of the bearing axis.
3.1.6 rotational stop—a feature that prevents relative rotation between two articulating joint surfaces beyond a specific angle of
rotation or creates resistance to rotation beyond a specific angel of rotation.
3.1.7 superior articulating interfaces—any interface in which relative motion occurs between the topside of the mobile bearing
component and the femoral bearing component.
4. Significance and Use
4.1 Fundamental aspects of this practice include the use of dynamic rotational force an
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.