ASTM D3454-97
(Test Method)Standard Test Method for Radium-226 in Water
Standard Test Method for Radium-226 in Water
SCOPE
1.1 This test method covers the measurement of soluble, suspended, and total radium-226 in water in concentrations above 3.7 X 10 -3 Bq/L. This test method is not applicable to the measurement of other radium isotopes.
1.2 This test method may be used for quantitative measurements by calibrating with a radium-226 standard, or for relative measurements by comparing the measurements made with each other.
1.3 This test method does not meet the current requirements of Practice D 2777.
1.4 The values stated in SI units are to be regarded as the standard. The inch-pound units given in parentheses are for information only.
1.5 Hydrofluoric acid (HF) is very hazardous and should be used in a well-ventilated hood. Wear rubber gloves, safety glasses or goggles, and a laboratory coat. Avoid breathing any HF fumes. Clean up all spills promptly and wash thoroughly after using HF.
1.6 This standard does not purport to address all of the other safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation:D3454–97
Standard Test Method for
Radium-226 in Water
This standard is issued under the fixed designation D3454; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 This test method covers the measurement of soluble, 3.1 Definitions—For definitions of terms used in this test
suspended, and total radium-226 in water in concentrations method, refer to Terminology D1129, and to other published
−3
above 3.7 310 Bq/L. This test method is not applicable to glossaries.
the measurement of other radium isotopes.
4. Summary of Test Method
1.2 This test method may be used for quantitative measure-
4.1 This test method is based on the emanation and
mentsbycalibratingwitharadium-226standard,orforrelative
measurements by comparing the measurements made with scintillationcountingofradon-222,agaseousdaughterproduct
of radium-226, from a solution.
each other.
1.3 Thistestmethoddoesnotmeetthecurrentrequirements 4.2 Radium-226 is collected from water by coprecipitation
on a relatively large amount of barium sulfate. The barium-
of Practice D2777.
1.4 The values stated in SI units are to be regarded as the radiumsulfateisdecomposedbyfumingwithphosphoricacid,
and the resulting glassy melt is dissolved by evaporation with
standard. The inch-pound units given in parentheses are for
information only. dilute hydrochloric acid to form soluble barium-radium phos-
phatesandchlorides.Thesesaltsaredissolvedandthesolution
1.5 Hydrofluoric acid (HF) is very hazardous and should be
used in a well-ventilated hood. Wear rubber gloves, safety is stored for ingrowth of radon-222. After a suitable ingrowth
period, the radon gas is removed from the solution by purging
glasses or goggles, and a laboratory coat.Avoid breathing any
HF fumes. Clean up all spills promptly and wash thoroughly with gas and transferred to a scintillation counting chamber.
About 4 h after radon-222 collection, the scintillation chamber
after using HF.
1.6 This standard does not purport to address all of the is counted for alpha activity. The radium-226 concentration is
calculated from the alpha count rate of radon-222 and its
other safety concerns, if any, associated with its use. It is the
immediate daughters. The radioactive decay characteristics of
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- radium-226 and its immediate decay progeny are listed in
Table 1.
bility of regulatory limitations prior to use.
2. Referenced Documents 5. Significance and Use
5.1 Themostprevalentofthefiveradiumisotopesinground
2.1 ASTM Standards:
D 1129 Terminology Relating to Water water, having a half life greater than one day, are radium-226
and radium-228. These two isotopes also present the greatest
D 1193 Specification for Reagent Water
D 2777 Practice for Determination of Precision and Bias health risk compared to the other naturally occurring nuclides
of equal concentrations if ingested via the water pathway.
Applicable Methods of Committee D-19 on Water
D 3370 Practices for Sampling Water from Closed Con- 5.2 Although primarily utilized on a water medium, this
technique may be applicable for the measurement of the
duits
D 3649 Practice for High-Resolution Gamma-Ray Spec- radium-226 content of any media once the medium has been
completely decomposed and put into an aqueous solution.
trometry of Water
1 4
This test method is under the jurisdiction ofASTM Committee D 19 on Water American National Standard Glossary of Terms in Nuclear Science and
andisthedirectresponsibilityofSubcommitteeD19.04onMethodsofRadiochemi- Technology, N1.1-1967.
cal Analysis. This test method is based on a previously published method by Rushing, D.E.,
Current edition approved Aug. 10, 1997. Published February 1998. Originally Garcia, W.J., and Clark, D.A. “The Analysis of Effluents and Environmental
published as D3454–75T. Last previous edition D3454–91. Samples from Uranium Mills and of Biological Samples for Radium, Polonium and
Annual Book of ASTM Standards, Vol 11.01. Uranium,” Radiological Health and Safety in Mining and Milling of Nuclear
Annual Book of ASTM Standards, Vol 11.02. Materials, Vol. II, IAEA, Vienna, Austria, 1964), p. 187.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D3454–97
TABLE 1 Radioactive Decay Characteristics of Radium-226 and
Its Daughters
Radionuclide Half-life Mode of Decay
Radium-226 1602 years a
Radon-222 3.82 days a
Polonium-218 3.05 min a
Lead-214 26.8 min b, g
Bismuth-214 19.7 min b, g
Polonium-214 164 µ s a
Lead-210 22.3 years b, g
5.3 Thegeneralmethodologyandbasisofthistechniqueare
similar to the methodology “Radium-226 in Drinking Water
(Radon Emanation Technique)” as described in the document
EPA-600//4-80-032.
6. Interferences
6.1 Onlythegaseousalpha-emittingradionuclidesinterfere,
namely, radon-219 and radon-220. Their half lives are 3.9 and
54.5 s respectively; their presence indicates the presence of
their parents, radium-223 and radium-224. These short-lived
radonisotopesdecaybeforetheradon-222iscounted;itistheir
alpha-emitting decay products that would interfere. These
interferences are very rare in water samples but are frequently
observed in certain uranium mill effluents.
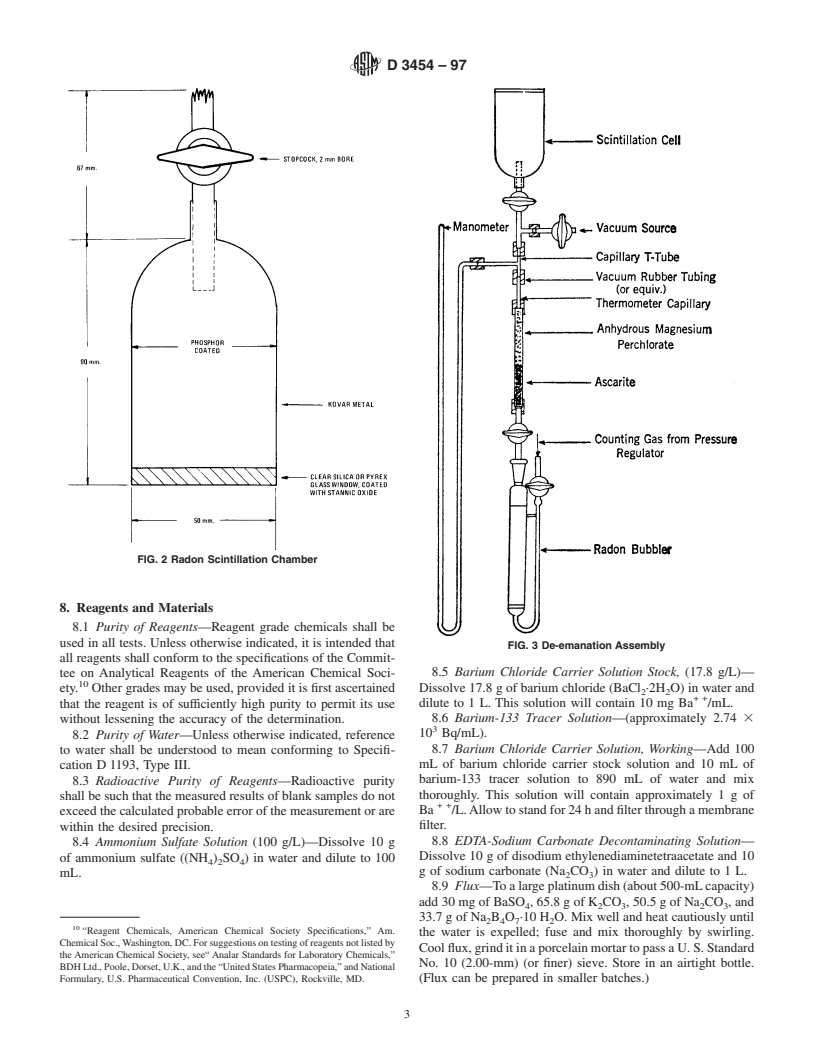
7. Apparatus
7.1 Radon Bubbler (Fig. 1).
7.2 Radon Scintillation Chamber (also known as Lucas
Cell) (Fig. 2).
7.3 Manometer, open-end capillary tube or vacuum gage
havingavolumewhichissmallcomparedtothevolumeofthe
FIG. 1 Radon Bubbler
scintillation chamber, 0,−760 mm Hg (Fig. 3).
7.4 Gas Purification Tube, 7 to 8 mm outside diameter
ascertained by determining a plateau using radon-222 in the
standard wall glass tubing, 100 mm long, constricted at lower
scintillation chamber as the alpha source. The slope of the
end to hold a glass wool plug (Fig. 3). The upper half of the
plateau should not exceed 2%/100 V. The counter and the
tube is filled with magnesium perchlorate and the lower half
with a sodium hydrate-asbestos absorbent. scintillation chamber should be calibrated and used as a unit
when more than one counter is available. The background
7.5 Scintillation Counter Assembly, consisting of a 50 mm
(2 in.) or more in diameter photomultiplier tube mounted in a counting rate for the counter assembly without the scintillation
chamber should range from 0.00 to 0.03 cpm.
light-tighthousingandcoupledtotheappropriatepreamplifier,
high-voltage supply, and scaler. A high-voltage safety switch 7.6 Membrane Filters, 0.45-µm pore size.
7.7 Silicone Grease, high-vacuum, for bubbler stopcocks.
should open automatically when the light cover is removed to
avoid damage to the photomultiplier tube. The preamplifier 7.8 Platinum Ware,crucibles,20to30mL,andone500-mL
capacity dish. Platinum ware is cleaned by immersing and
should incorporate a variable gain adjustment. The counter
rotating in a molten bath of potassium pyrosulfate, removing,
should be equipped with a flexible ground wire which is
cooling, and rinsing in hot tap water, digesting in hot HCl
attached to the chassis photomultiplier tube by means of an
(1+1), rinsing in water, and finally flaming over a burner.
alligator clip or similar device. The operating voltage is
7.9 Laboratory Glassware—Glassware may be decontami-
nated before and between uses by heating for1hinEDTA-
“Radium-226 in Drinking Water (Radon Emanation Technique),” Prescribed
Na CO decontaminating solution at 90 to 100°C, then rinsing
2 3
Procedures for Measurement of Radioactivity in Drinking Water, August 1980.
in water, in (1+11) HCl and again in water.
Available from Corning Glass Works, Special Sales Section, Corning, N.Y.
11830.
Available from W. H. Johnston Laboratories, 3617 WoodlandAve., Baltimore,
MD21215,andRockyMountainScientificGlassBlowingCo.,4990E.AsburyAve. Type HAWP(Millipore filter Corp., Bedford, MA) has been found satisfactory.
Denver, CO 80222. An equivalent may be used.
D3454–97
FIG. 2 Radon Scintillation Chamber
8. Reagents and Materials
8.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
FIG. 3 De-emanation Assembly
all reagents shall conform to the specifications of the Commit-
tee on Analytical Reagents of the American Chemical Soci- 8.5 Barium Chloride Carrier Solution Stock, (17.8 g/L)—
Dissolve 17.8 g of barium chloride (BaCl ·2H O) in water and
ety. Other grades may be used, provided it is first ascertained
2 2
++
that the reagent is of sufficiently high purity to permit its use dilute to 1 L. This solution will contain 10 mg Ba /mL.
8.6 Barium-133 Tracer Solution—(approximately 2.74 3
without lessening the accuracy of the determination.
8.2 Purity of Water—Unless otherwise indicated, reference 10 Bq/mL).
8.7 Barium Chloride Carrier Solution, Working—Add 100
to water shall be understood to mean conforming to Specifi-
cation D1193, Type III. mL of barium chloride carrier stock solution and 10 mL of
barium-133 tracer solution to 890 mL of water and mix
8.3 Radioactive Purity of Reagents—Radioactive purity
thoroughly. This solution will contain approximately1gof
shall be such that the measured results of blank samples do not
++
Ba /L.Allowtostandfor24handfilterthroughamembrane
exceedthecalculatedprobableerrorofthemeasurementorare
filter.
within the desired precision.
8.8 EDTA-Sodium Carbonate Decontaminating Solution—
8.4 Ammonium Sulfate Solution (100 g/L)—Dissolve 10 g
Dissolve 10 g of disodium ethylenediaminetetraacetate and 10
of ammonium sulfate ((NH ) SO ) in water and dilute to 100
4 2 4
g of sodium carbonate (Na CO ) in water and dilute to 1 L.
mL.
2 3
8.9 Flux—Toalargeplatinumdish(about500-mLcapacity)
add 30 mg of BaSO,65.8gofK CO,50.5gofNa CO , and
4 2 3 2 3
33.7gofNa B O ·10 H O. Mix well and heat cautiously until
2 4 7 2
“Reagent Chemicals, American Chemical Society Specifications,” Am.
the water is expelled; fuse and mix thoroughly by swirling.
ChemicalSoc.,Washington,DC.Forsuggestionsontestingofreagentsnotlistedby
Coolflux,grinditinaporcelainmortartopassaU.S.Standard
theAmerican Chemical Society, see“Analar Standards for Laboratory Chemicals,”
No. 10 (2.00-mm) (or finer) sieve. Store in an airtight bottle.
BDHLtd.,Poole,Dorset,U.K.,andthe“UnitedStatesPharmacopeia,”andNational
Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville, MD. (Flux can be prepared in smaller batches.)
D3454–97
8.10 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro- date and time and store the bubbler preferably for 2 to 3 weeks
chloric acid (HCl). before collecting and counting the radon-222.
8.11 Hydrochloric Acid Solution (1+1)—Mix 1 volume of
10.4 Attach a scintillation chamber as shown in Fig. 3;
concentrated HCl (sp gr 1.19) with 1 volume of water. substitute a glass tube with a stopcock for the bubbler so that
8.12 Hydrochloric Acid Solution (1+11)—Mix 1 volume
theheliumgascanbeturnedonandoffconveniently.Openthe
of concentrated HCl (sp gr 1.19) with 11 volumes of water. stopcockonthescintillationchamber;closethestopcocktothe
8.13 Hydrochloric Acid Solution (1+49)—Mix 1 volume
gas and gradually open the stopcock to vacuum source to
of concentrated HCl (sp gr 1.19) with 49 volumes of water. evacuatethecell.Closethestopcocktothevacuumsourceand
8.14 Hydrochloric Acid Solution (1+119)—Mix 1 volume
check the manometer reading for 2 min to test the system,
of concentrated HCl (sp gr 1.19) with 119 volumes of water. especially the scintillation chamber for leaks. If leaks are
8.15 Hydrofluoric Acid (sp gr 1.15)—Concentrated hydrof- detected they should be identified and sealed.
luoric acid (HF). Use extreme caution. 10.5 Open the stopcock to the helium gas and allow the gas
8.16 Hydrogen Peroxide (1 + 9)—Mix 1 volume of
to enter the chamber slowly until atmospheric pressure is
H O (30%) with 9 volumes of water. reached. Close all the stopcocks.
2 2
8.17 Magnesium Perchlorate—Anhydrous magnesium per-
10.6 Place the scintillation chamber on the photomultiplier
chlorate Mg(ClO ) .
tube (in a light-tight housing), wait 10 min, and obtain a
4 2
8.18 Phosphoric Acid (sp gr 1.69)—Concentrated phospho-
background count rate (preferably over a period of at least 100
ric acid (H PO ).
min). Phototube must not be exposed to external light with the
3 4
8.19 Radium Standard Solution (0.37 Bq/mL).
high voltage applied.
8.20 Sodium Hydroxide-Coated Silicate Absorbent, Propri-
10.7 Withthescintillationchamberandbubblerinpositions
etary, 8 to 20 mesh.
indicated in Fig. 3 and all stopcocks closed, open the stopcock
8.21 Sulfuric Acid (sp gr 1.84)—Concentrated sulfuric acid
to vacuum and then to the scintillation chamber. Evacuate the
(H SO ).
2 4 scintillation cell and the gas purification system. Close the
8.22 Sulfuric Acid Solution (1+359)—Mix 1 volume of
stopcock to vacuum and check for leaks as in 10.4.
concentrated H SO (sp gr 1.84) with 359 volumes of water.
2 4 10.8 Adjust the helium regulator (diaphragm) valve so that
This solution is 0.1 N. Slowly add acid to water.
averyslowstreamofgaswillflowwiththeneedlevalveopen.
8.23 Helium, in a high-pressure cylinder with a two-stage
Attach the helium supply to the inlet of the bubbler.
pressure regulator and needle valve.
10.9 Very cautiously open the bubbler outlet stopcock to
equalize pressure and transfer all or most of the fluid in the
9. Sampling
inlet side arm to the bubbler chamber.
9.1 Collect the sample in accordance with the applicable 10.10 Close the outlet stopcock and very cautiously open
standards as described in Practices D3370. the inlet stopcock to flush remaining fluid from the side arm
and fritted disk. Close the inlet stopcock.
10. Calibration and Standardization
10.11 Repeat steps 10.9 and 10.10 several times to obtain
more nearly equal pressure on the two sides of the bubbler.
10.1 Close the inlet stopcock of a bubbler, (Note 1) add 5
10.12 With the outlet stopcock fully open, cautiously open
mLof BaCl ·2H O carrier solution, 1 mLof concentrated HCl
2 2
theinletstopcocksothattheflowofgasproducesafrothafew
(spgr1.19),3mL(1.1Bq)ofstandardradiumsolutionandfill
2 3
millimetres thick at the surface of bubbler solution. Maintain
the bubbler ⁄3 to ⁄4 full with water.
the flow rate by adjusting the pressure with the regulator valve
NOTE 1—Before using, test bubblers by placing about 10 mL of water
andcontinuede-emanationuntilthepres
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.