ASTM F787-82(1991)
(Specification)Specification for Metallic Nail-Plate Appliances (Withdrawn 1998)
Specification for Metallic Nail-Plate Appliances (Withdrawn 1998)
General Information
Standards Content (Sample)
ASTM F?8? 82 = 0?595l10 0509823 138
Designation: F 787 - 82 (Reapproved 1991)
Standard Specification for
Metallic Nail-Plate Appliances’
This standard is issued under the fixed designation F 787; the number immediately following the designation indicates the year of
o&al adoption or, in the case of revision, the year of last revision. A number in parenthesa iadicates the year of last reapproval. A
superscript epaiioa (e) indicates an editorid sina the iast revision or reapproval.
.
3. Materials and Manufacture
1. scope
3.1 Nail plates shaii be fabricated from material con-
1.1 This specification covers functional dimensidns, toler-
forming to one of the following ASTM Specifications: F 55,
ances and materials for nad-plates used in the treatment of
F 56, F 67, F 75, F 90, F 136, F 138, and F 139.
fractures.
3.1.1 Nail plates of forged Specification F 136 shali meet
1:2 The values stated in inch-pound units are to be
the requirements of Specification F 620.
regarded as the standard.
3.1.2 Nail plates of forged Specification F 55 or specifica-
tion F 138 shall meet the requirements of Specification F 62 1.
2. Referenced Documents 2
4. Períormance Considerations
2.1 ASTM Standards:
4.1 Nail plates may be tested using Practice F 384.
F 55 Specification for Stainless Steel Bar and Wire for
4.2 Factors considered to be important, but for which
Surgid impiants’
values and test methods have not been established, are bend-
F 56 Specification for Stainless Steel Sheet and Strip for
ing strength, fatigue strength, ductiüty, and bending rigidity.
Surgid Impiants2
5. Dimensions, Mass, and Permissible Variations
F 67 Specification for Unalloyed Titanium for Surgical
Implant Applications’
5.1 Nail plates shail be fabricated in accordance with the
F 75 Specification for Cast Colbalt-Chromium-Molybde
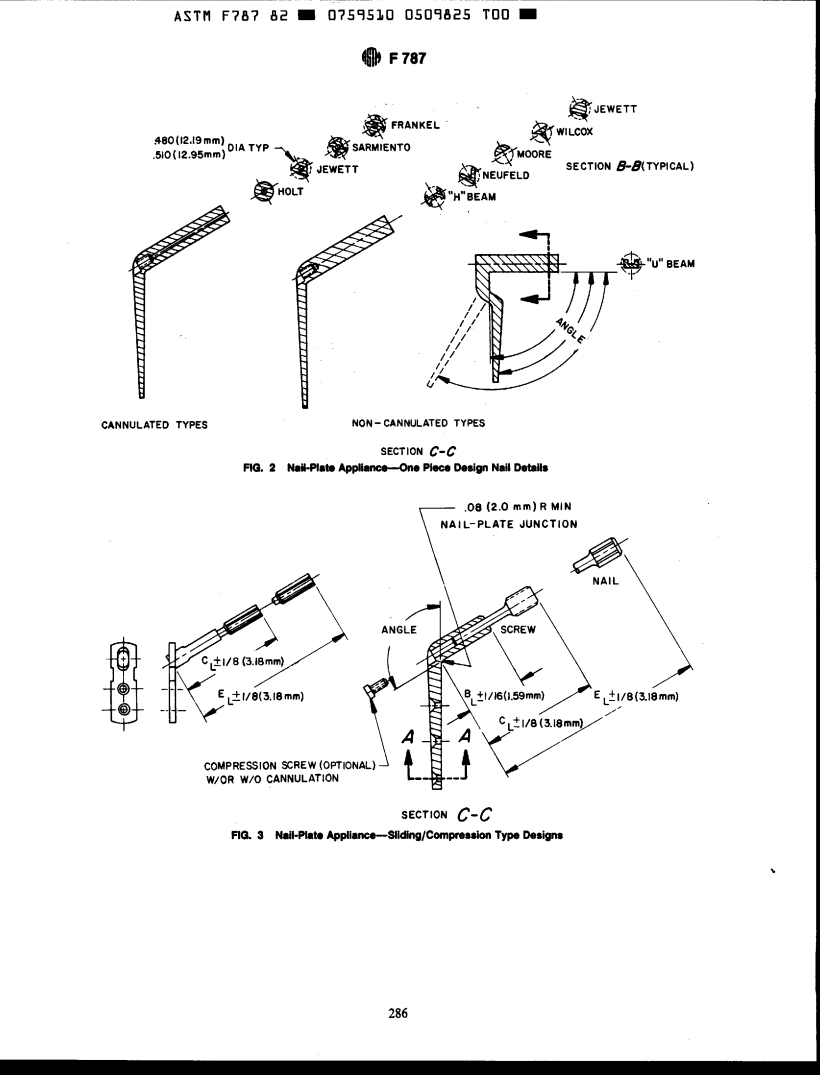
dimensions and tolerances shown in Figs. 1, 2, 3, 4, and 5.
num Alloy for Surgid Implant Applications*
Notes for Fig. 1 apply to Figs. 3 and 4, also.
F 86 Practice for Surface Preparation and Marking of 5.2 Nail plates shall have no sharp exterior edges, except
Metallic Surgid Implants’ where a sharp edge provides a functional purpose.
F 90 Specification for Wrought Cobalt-Chromium-Tung- 5.3 Nail plates shail have surfaces prepared and shall be
sten-Nickel Alloy for Surgid Implant Applications’ marked using a method specified in accordance with Practice
F 136 Specification for Wrought Titanium 6Al-4V ELI F 86.
Alloy for Surgid Implant Applications’ 5.3.1 Markings on the nail plate shall identis. the manu-
F 138 Specification for Stainless Steel Bars and Wire for facturer or distributor and shali be near the ends of the plate
and away from the most highly stressed area, where possible.
Surgical Implants (Special Quality)’
5.4 Dimensions and tolerances for screw holes and screw
F 139 Specification for Stainless Steel Sheet and Strip for
slots in nail plates shali conform to Practice F 367, for use
Surgid Implants (Special Quality?
with bone screws conforming to Specification F 543, Table 1.
F 367 Specification for Holes and Slots for Inch Cortid
Bone Screws’
6. Packaging and Labeling
F 384 Practice for Static Bend Testing of Nail Plates’
6.1 Packaging shail be adequate to protect the nail plate
F 543 Specification for Cortical Bone Screws’
during shipment.
F 565 Practice for Care and Handling of Orthopedic
6.2 Labeling for nail plates shall include:
Implants and Instruments’
6.2.1 Product name,
F 620 Specification for Titanium 6A1-4V ELI Alloy
6.2.2 Size, on the immediate container,
Forgings for Surgical Implants’
6.2.2.1 Plate length
F 621 Specification for Stainless Steel Forgings for Sur-
6.2.2.2 Plate width,
gid impiants’
6.2.2.3 Plate thickness,
6.2.2.4 Nail length,
6.2.2.5 Number of screw holes,
I This specification is under the jurisdiction of ASTM Committee F-4 on
Medical and Surgical Materials and Devices and is the direct responsibility of
6.2.2.6 Size of screw holes, and
Subcommittee Fû4.03 on orthopaedics.
*
6.2.3 ASTM Material Specification Designation Number.
Current edition approved Aug. 27, 1982. Published January, 1983. Onginally
6.3 Nail plates shall be med for and handled in accord-
published as F 787 - 82. Last previous edition F 787 - 82.
* Annual Book of ASTA4 Sthrak, Vol 13.0 I. ance with Practice F 565.
284
---------------------- Page: 1 ----------------------
ASTM F787 82 = 0759530 O509824 074 W
@ F787
.350 (8.89mm) DIA C'BORE MIN TO 7SOAFULL LAND MIN
1/4-20 UNC-28 MOD THREAD
.265(674mm) DEEP MIN
.221(5.61mm)MINOR DIA MOO MAX
.5i O{ 12.95 m m ) DIA
y .480 (1219mm)
.625(Iá88mm) MI
.lO9(277mm)DlA MIN
CANNULATION (OPTTIONAL)
JUNCTION .120( 3.05 mm) R MIN
SEE NOTE 2
- 1/16 (1.59mm)
J
SEE NOTE I
SECTION c-c
.750(19.05mrn) TO 1.000(25.4Omm) ~.010(0.25mm) TYPICAL
\
SEE FlG.2 FOR SECTION 8-B
SEE FIG5 FOR SECTION A-A
NOTE l-End of plate may opaonaiiy have a full radius or radiused come*i.
NOTE 2-Locatlo
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.