ASTM E1641-99
(Test Method)Standard Test Method for Decomposition Kinetics by Thermogravimetry
Standard Test Method for Decomposition Kinetics by Thermogravimetry
SCOPE
1.1 This test method covers determination of the kinetic parameters, Arrhenius activation energy, and pre-exponential factor by thermogravimetry, based on the assumption that the decomposition obeys first-order kinetics.
1.2 This test method is generally applicable to materials with well-defined decomposition profiles, namely, a smooth, continuous mass change with a single maximum rate.
1.3 This test method is normally applicable to decomposition occurring in the range from 400 to 1300K (100 to 1000°C). The temperature range may be extended depending on the instrumentation used.
1.4 Computer or electronic-based instruments, techniques, or data treatment equivalent to this test method may also be used. Users of this test method are expressly advised that all such instruments or techniques may not be equivalent. It is the responsibility of the user of this test method to determine the necessary equivalency prior to use. Only the manual procedures described in this test method are to be considered valid in the case of dispute.
1.5 The values stated in SI units are to be regarded as the standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E 1641 – 99
Standard Test Method for
Decomposition Kinetics by Thermogravimetry
This standard is issued under the fixed designation E 1641; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope E 1877 Practice for Calculating Thermal Endurance of Ma-
terials From Thermogravimetric Decomposition Data
1.1 This test method covers determination of the kinetic
parameters, Arrhenius activation energy, and preexponential
3. Terminology
factor by thermogravimetry, based on the assumption that the
3.1 Technical terms used in this test method are defined in
decomposition obeys first-order kinetics.
Terminologies E 473 and E 1142.
1.2 This test method is generally applicable to materials
with well-defined decomposition profiles, namely, a smooth,
4. Summary of Test Method
continuous mass change with a single maximum rate.
4.1 This test method consists of heating a series of four or
1.3 This test method is normally applicable to decomposi-
more test specimens, taken from the original sample, each at a
tion occurring in the range from 400 to 1300K (100 to
different heating rate between 1 and 10K/min, through their
1000°C). The temperature range may be extended depending
decomposition region. The specimen mass is recorded continu-
on the instrumentation used.
ously as a function of temperature. The temperatures for
1.4 Computer or electronic-based instruments, techniques,
constant conversion are determined from the resultant mass
or data treatment equivalent to this test method may also be
loss curves. The Arrhenius activation energy is then determined
used.
from a plot of the logarithm of heating rate versus the
NOTE 1—Users of this test method are expressly advised that all such
reciprocal of the absolute temperature at constant conversion
instruments or techniques may not be equivalent. It is the responsibility of
level. This activation energy may then be used to calculate
the user of this test method to determine the necessary equivalency prior
thermal endurance and an estimate of the lifetime of the
to use. Only the manual procedures described in this test method are to be
material at a certain temperature.
considered valid in the case of dispute.
1.5 The values stated in SI units are to be regarded as the
5. Significance and Use
standard.
5.1 Thermogravimetry provides a rapid method for deter-
1.6 This standard does not purport to address all of the
mining the temperature-decomposition profile of a material.
safety concerns, if any, associated with its use. It is the
5.2 This test method can be used for estimating lifetimes of
responsibility of the user of this standard to establish appro-
materials, using Test Method E 1877 provided that a relation-
priate safety and health practices and determine the applica-
ship has been established between the thermal endurance test
bility of regulatory limitations prior to use.
results and actual lifetime tests.
2. Referenced Documents
6. Apparatus
2.1 ASTM Standards:
6.1 The essential equipment required to provide the mini-
E 473 Terminology Relating to Thermal Analysis
mum thermogravimetric analytical capability of this test
E 1142 Terminology Relating to Thermophysical Proper-
method includes:
ties
6.1.1 A thermobalance, composed of (a)a furnace to
E 1582 Practice for Calibration of Temperature Scale for
provide uniform controlled heating of a specimen at a constant
Thermogravimetry
rate within the temperature range from ambient to 900 K; (b)
a temperature sensor to provide an indication of the specimen/
furnace temperature to 60.1 K; (c)an electrobalance to
continuously measure the specimen mass with a minimum
This test method is under the jurisdiction of ASTM Committee E-37 on
Thermal Measurements and is the direct responsibility of Subcommittee E37.01 on
capacity of 20 mg and a sensitivity of 650 μg; and (d) a means
Test Methods.
of sustaining the specimen/container under atmospheric con-
Current edition approved Dec. 10, 1999. Published March 2000. Originally
trol of an inert or reactive purge gas of 99.99 % purity at a rate
published as E 1641 – 94. Last previous edition E 1641 – 98.
Annual Book of ASTM Standards, Vol 14.02. of 20 to 50 6 5 mL/min.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E 1641
6.1.2 A temperature controller, capable of executing a 8.3 Certain materials require more sophisticated condition-
specific temperature program by operating the furnace between ing, such as maintaining the sample at a specified room
selected temperature limits at a rate of temperature change temperature and relative humidity for an extended period of
between 1 and 10 K/min to within 60.1 K/min. time. Such conditioning may be conducted, but procedural
6.1.3 A recording device, either analog or digital, capable of details shall be included in the report.
recording and displaying the change in mass with of 650 μg
and temperature with a resolution of 0.1 K.
9. Procedure
6.1.4 Containers (pans, crucibles, and so forth) which are
9.1 Calibrate the instrument mass balance in accordance
inert to the specimen and which will remain dimensionally
with the procedure recommended for the instrument in use.
stable over the temperature range from ambient to 900 K.
9.2 Place the temperature sensor within 2 mm of the outside
6.2 High-Purity (99.99 %) Nitrogen Supply, for purge gas.
of the specimen holder. Care must be taken to ensure that the
specimen holder is not touched in any way by the sensor and
NOTE 2—Other atmospheres may be used but shall be specified.
that it is not moved after temperature calibration.
7. Precautions
9.3 Maintain a constant flow rate of purge gas in the range
from 20 to 50 mL/min throughout the experiment.
7.1 It is essential that the samples be representative since
milligram quantities of specimen are to be used.
NOTE 5—In the case of samples that may be sensitive to oxidative
7.2 The value of the calculated activation energy is inde-
degradation, it will be necessary to maintain inert gas purging for a time
pendent of reaction order in the early stages of decomposition.
sufficient to ensure that all residual oxygen is removed from the system
This assumption does not hold for the later stages and shall be prior to the start of the temperature program. It may be necessary to
evacuate the system prior to initiating inert gas purging for some
used with caution. An upper limit of 10 % decomposition is
instruments.
suggested, although 20 % is justified in certain cases. It is
strongly suggested that calculations be made at several differ-
9.4 Calibrate the instrument furnace temperature in accor-
ent levels of decomposition, for example, 5, 10, 15, and 20 %.
dance with the calibration procedure in Practice E 1582 using
Variations in the results among these determinations could
the same heating rate, purge gas, and flow rate to be used for
indicate the inapplicability of one of them. For instance,
the specimens. The temperature calibration shall be performed
volatile, low-level impurities would affect the results of the
both prior to every change in heating rate and at that heating
lowest conversion determination more than those at higher
rate.
conversions. Consistent results for all conversions validate the
9.5 Place 3 6 1 mg of the specimen under test into a clean,
method for the range of conversions examined.
tared instrument specimen holder. Other specimen sizes may
7.3 Toxic or corrosive effluents, or both, may be released
be used but shall be indicated in the report.
during the heating process and may be harmful to the personnel
NOTE 6—The specimen holder should be tared in the fully assembled
or apparatus.
system, with the purge gas flowing.
NOTE 7—Powdered or granular specimens should be distributed evenly
8. Sampling
over the specimen holder so as to maximize the exposed surface. A
8.1 Powdered or granular specimens, which have a high
one-grain thick layer would be optimal.
surface-to-volume ratio, are preferred, although films, fibers,
9.6 Equilibrate the specimen at a temperature, in kelvins
and fabrics may be used providing that care is taken to make all
(K), of ten times the heating rate in kelvins per minute below
of the specimens uniform in size and shape. Under circum-
the known decomposition temperature. If the percentage mass
stances in which material parts are available, the specimens
loss is to be recorded, establish zero percent loss at this time.
should be prepared by filing or rasping the part. All specimens
should be mixed thoroughly prior to sampling if possible, and
NOTE 8—If zero percent mass loss is established at the time at which
the specimen is placed into the instrument, the specimen mass at the
they should be sampled by removing portions from various
equilibration temperature can be greater than 100 % due to buoyancy
parts of the container. These portions should in turn be
effects. A blank should be run for accurate determination of the buoyancy
combined and mixed well to ensure a representative sample for
effect throughout the temperature range of the experiment. The blank can
the determination.
be a piece of platinum of approximately the same volume as the specimen.
The balance drift at any temperature can be determined in this manner.
NOTE 3—Care should be exercised during sample preparation to avoid
contamination.
9.7 Heat the specimen at a constant rate through the
NOTE 4—The specimen size and surface-to-volume ratio are known to
decomposition profile until a constant mass is obtained or the
affect the results of this test. A narrow range of specimen sizes should be
temperature is well beyond the useful temperature range of the
used, as noted in 9.5. Uniformity in particle size can be achieved, without
material tested. Record the accompanying thermal curve, with
the loss of volatiles, by using a liquid nitrogen mill to grind the sample to
a fine powder. To prevent the condensation of moisture, the mill should be
mass or percentage mass loss displayed on the ordinate and
opened only after returning fully to ambient temperature, or the operation
specimen temperature on the abscissa.
should be performed in a glove box filled with dry gas.
9.8 Once the decomposition of the test specimen is com-
8.2 In the absence of other information, the samples are plete, cool the instrument to room temperature, remove, clean,
assumed to be analyzed as received except for the mechanical and replace the specimen holder, and retare the instrument in
treatment noted in 8.1. If some heat treatment, such as drying, preparation for additional experiments. Use the same specimen
is applied to the sample prior to analysis, this treatment and any holder for the entire series of runs to eliminate buoyancy
resulting mass loss must be noted in the report. problems.
E 1641
NOTE 12—An apparent nonlinearity may result from erroneous deter-
9.9 Repeat the procedures described in 9.4-9.8 at three
minations. It is recommended that any nonlinear points be repeated for
additional heating rates covering the range from 1 to 10 K/min.
verification.
Other heating rates, and more than four, may be used but shall
be noted in the report.
10.3 Using the least-squares method fit a straight line to
these data without weighing factors, and determine the slope
NOTE 9—The use of heating rates greater than 10 K/min affects both the
precision of the temperature measurement and the kinetics of the
D~logb!/D~1/T!
decomposition. Diffusion of volatiles from the sample may become the
rate-controlling process at high heating rates.
NOTE 13—If the values obtained from this test method are to be used in
Test Method E 1877, an estimation of the uncertainty for activation energy
10. Calculation
(E) and preexponential factor (A) is required. These uncertainties may be
10.1 From each of the thermal curves obtained in 9.5-9.9, derived from the uncertainty in the slope value of m5D (log b)/D (1/T).
If the calculation tool used to obtain the slope of the straight line provides
determine the absolute temperature at constant conversion, a,
an estimation of uncertainty in the determined slope (dm), record it.
for each of the constant conversion values to be used in the
Otherwise, the uncertainty in the slope may be obtained using the
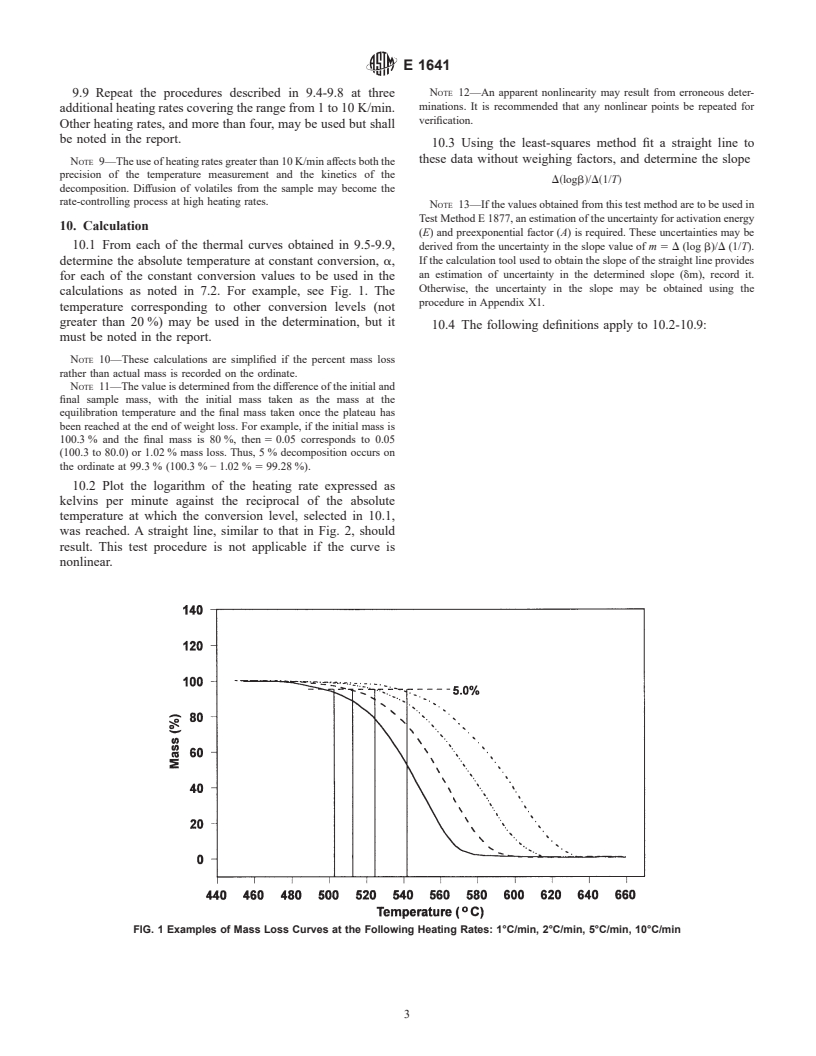
calculations as noted in 7.2. For example, see Fig. 1. The
procedure in Appendix X1.
temperature corresponding to other conversion levels (not
greater than 20 %) may be used in the determination, but it
10.4 The following definitions apply to 10.2-10.9:
must be noted in the report.
NOTE 10—These calculations are simplified if the percent mass loss
rather than actual mass is recorded on the ordinate.
NOTE 11—The value is determined from the difference of the initial and
final sample mass, with the initial mass taken as the mass at the
equilibration temperature and the final mass taken once the plateau has
been reached at the end of weight loss. For example, if the initial mass is
100.3 % and the final mass is 80 %, then 5 0.05 corresponds to 0.05
(100.3 to 80.0) or 1.02 % mass loss. Thus, 5 % decomposition occurs on
the ordinate at 99.3 % (100.3 % − 1.02 % 5 99.28 %).
10.2 Plot the logarithm of the heating rate expressed as
kelvins per minute against the reciprocal of the absolute
temperature at which the conversion level, selected in 10.1,
was reached. A straight line, similar to that in Fig. 2, should
result. This test procedure is not applicable if the curve is
nonlinear.
FIG. 1 Examples of Mass Loss Curves at the Following Heating Rates: 1°C/min, 2°C/min, 5°C/min, 10°C/min
E 1641
FIG. 2 Arrhenius Plot of Heating Rate, Temperature of Constant Conversion Data
10.9 Select the mass loss curve for the heating rate nearest
the midpoint of the experimental heating rates, and calculate
E 5 refined Arrhenius activation energy,
the pre-exponential factor, A, using Eq 2 (1, 2, 3, 4) and the
J/mol,
−1
A 5 pre-exponential factor, min , value of the exponent, a, obtained from Table 1 for the refined
R 5 gas constant, 8.314 J/(mol·K), value of E /RT determined in 10.7.
e c
D(logb)/D(1/T) 5 slope of the line obtained in 10.2,
a
A52~b8/E !*R*ln ~12a!*10 (2)
r
b5 heating rate, K/min,
b8 5 heating rate nearest the midpoint of the
NOTE 14—This mathematical treatment to solve for E and A has been
r
tailored specifically to make it possible to perform by hand. Commercial
experimental heating rates, K/min,
kinetics software may use other nume
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.