ASTM E3251-20
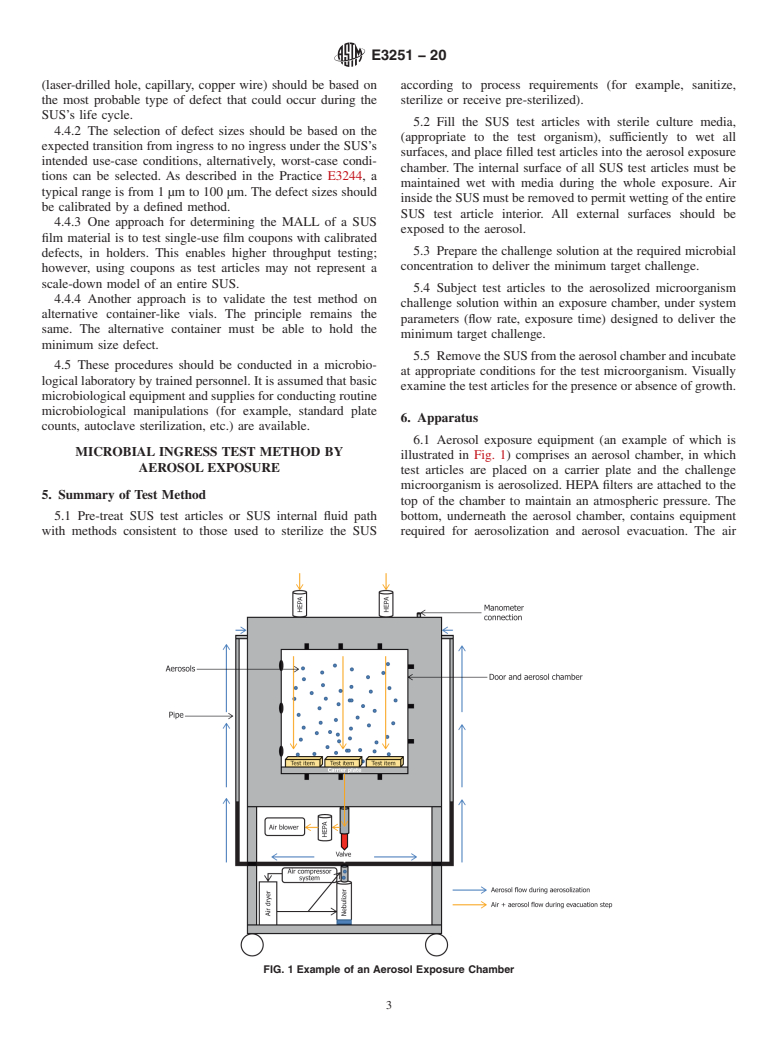
(Test Method)Standard Test Method for Microbial Ingress Testing on Single-Use Systems
Standard Test Method for Microbial Ingress Testing on Single-Use Systems
SIGNIFICANCE AND USE
4.1 Single-use systems (SUSs) used for biopharmaceutical manufacturing must maintain sterility and product quality of the fluid inside. Such articles or systems should therefore be validated as providing an effective barrier against microbial ingress. The microbial barrier properties of a SUS may be demonstrated using deterministic physical tests that have been correlated to microbial integrity. Two test methods (aerosol exposure and immersion exposure) are described that can be used to demonstrate microbial integrity of a SUS or determine the MALL, the maximum defect size that does not allow microbial ingress, into a SUS.
4.2 It is important to note that the results of microbial ingress tests are heavily dependent on the conditions under which the test is performed and are not suitable for routine checking of a SUS due to the test’s destructive nature.
4.2.1 Any size defect may be forced to fail under sufficiently aggressive conditions (including a large enough sample size, high differential pressure, or high hydrostatic pressure, for example) that would not ordinarily reflect normal use conditions. Thus, it is necessary to clearly define the relevant conditions for a test through a risk assessment of both the actual SUS claims and its final use (Practice E3244). Once that is established, the size of defect that can be detected under those conditions can be determined, if required, using defined defects.
4.2.2 “Relevant conditions” refers to worse-case actual use conditions but does not mean that a SUS must be tested under theoretically absolute (extreme) “worst-case” conditions.
4.2.3 Testing may be performed on individual components or entire systems. Considerations for defining “relevant conditions” and testing design should be based on a risk assessment for the SUS intended use and should include:
4.2.3.1 A channel created by a defect or breach through the film thickness or through a seam or connection which must be filled with liquid to allow mic...
SCOPE
1.1 The microbial test method outlined in this document applies to microbial ingress risk assessment of a single-use system (SUS) or its individual components that require integrity testing either by the assembly supplier or the end user of the assembly based on a potential risk of a breach to the product or manufacturing process.
1.2 The aim of microbial ingress testing of sterile SUSs used in biopharmaceutical manufacturing is two-fold:
1.2.1 Firstly, it is used to evaluate the ability of a SUS fluid path to remain sterile after a SUS has been challenged by microbial exposure. Microbial exposure is achieved either by directly placing a SUS into a container of microbial challenge solution, or by delivering an aerosolized microbial challenge onto a SUS that is placed inside a test chamber designed to generate and deliver the aerosol. The choice of the test challenge organism should be justified based on a risk assessment of the SUS and conditions of use.
1.2.2 Additionally, microbial ingress testing can be used to determine the maximum allowable leakage limit (MALL) that does not allow microbial ingress under specific test conditions. The defect size that can be detected by specific physical integrity testing methods can be correlated to this MALL in order to claim microbial integrity. Test articles bearing calibrated defects over a range of dimensions, including up to a defect size expected to consistently allow microbial ingress as a positive control (defect-based positive control), may be tested to determine the MALL.
1.3 Both purposes for microbial ingress testing as described in 1.2.1 and 1.2.2 can either be conducted by liquid immersion or aerosol exposure. For the purpose described in 1.2.2, the type of exposure should be determined according to the SUS’s use-case conditions and a risk assessment.
1.4 The method used to create a breach, hole or defect in single-use film or in a SUS test article, as ...
General Information
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E3251 − 20
Standard Test Method for
1
Microbial Ingress Testing on Single-Use Systems
This standard is issued under the fixed designation E3251; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope given test article should be justified with the rationale of
sampling size to obtain a statistically meaningful effect (Prac-
1.1 The microbial test method outlined in this document
tice E3244). Determining the appropriate number of SUS test
applies to microbial ingress risk assessment of a single-use
articles will depend on a risk assessment of the SUS and the
system (SUS) or its individual components that require integ-
conditions of its use and is also outside of this document’s
rity testing either by the assembly supplier or the end user of
scope.
the assembly based on a potential risk of a breach to the
product or manufacturing process. 1.5 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this
1.2 The aim of microbial ingress testing of sterile SUSs
standard.
used in biopharmaceutical manufacturing is two-fold:
1.6 This standard does not purport to address all of the
1.2.1 Firstly, it is used to evaluate the ability of a SUS fluid
safety concerns, if any, associated with its use. It is the
path to remain sterile after a SUS has been challenged by
responsibility of the user of this standard to establish appro-
microbial exposure. Microbial exposure is achieved either by
priate safety, health, and environmental practices and deter-
directly placing a SUS into a container of microbial challenge
mine the applicability of regulatory limitations prior to use.
solution, or by delivering an aerosolized microbial challenge
1.7 This international standard was developed in accor-
onto a SUS that is placed inside a test chamber designed to
dance with internationally recognized principles on standard-
generate and deliver the aerosol. The choice of the test
ization established in the Decision on Principles for the
challenge organism should be justified based on a risk assess-
Development of International Standards, Guides and Recom-
ment of the SUS and conditions of use.
mendations issued by the World Trade Organization Technical
1.2.2 Additionally, microbial ingress testing can be used to
Barriers to Trade (TBT) Committee.
determine the maximum allowable leakage limit (MALL) that
does not allow microbial ingress under specific test conditions.
2. Referenced Documents
The defect size that can be detected by specific physical
2
2.1 ASTM Standards:
integrity testing methods can be correlated to this MALL in
order to claim microbial integrity. Test articles bearing cali- E3244 Practice for Integrity Assurance and Testing of
Single-Use Systems
brated defects over a range of dimensions, including up to a
defect size expected to consistently allow microbial ingress as 2.2 Other Documents:
USP <1207> Sterile Product Packaging — Integrity
a positive control (defect-based positive control), may be tested
3
to determine the MALL. Evaluation, 2016
4
ISO 15747 Plastic Containers for Intravenous Injections
1.3 Both purposes for microbial ingress testing as described
in 1.2.1 and 1.2.2 can either be conducted by liquid immersion
3. Terminology
or aerosol exposure. For the purpose described in 1.2.2, the
3.1 Definitions:
type of exposure should be determined according to the SUS’s
3.1.1 (calibrated) artificial defect, n—an artificial breach or
use-case conditions and a risk assessment.
defect (that is, laser-drilled hole, capillary) representing typical
1.4 The method used to create a breach, hole or defect in
failure modes, intentionally introduced into a test article.
single-use film or in a SUS test article, as well as the analytical
method used to physically characterize the defect size is
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
outside of the scope of this document. The sampling plan for a
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
1 3
This test method is under the jurisdiction of ASTM Committee E55 on Available from U.S. Pharmacopeial Convention (USP), 12601 Twinbrook
Manufacture of Pharmaceutical and Biopharmaceutical Products and is the direct Pkwy., Rockville, MD 20852-1790, http://www.usp.org.
4
responsibility of Subcommittee E55.04 on General Biopharmaceutical Standards. Available from International Organization for Standardization (ISO), ISO
Current edition approved May 1, 2020.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.