ASTM D6666-04(2019)
(Guide)Standard Guide for Evaluation of Aqueous Polymer Quenchants
Standard Guide for Evaluation of Aqueous Polymer Quenchants
SIGNIFICANCE AND USE
4.1 The significance and use of each test method will depend on the system in use and the purpose of the test method listed under Section 7. Use the most recent editions of the test methods.
SCOPE
1.1 This guide provides information, without specific limits, for selecting standard test methods for testing aqueous polymer quenchants for initial qualification, determining quality, and the effect of aging.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory requirements prior to use.
1.3 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D6666 − 04 (Reapproved 2019)
Standard Guide for
Evaluation of Aqueous Polymer Quenchants
This standard is issued under the fixed designation D6666; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope the Centrifuge Method (Laboratory Procedure)
D2624 Test Methods for Electrical Conductivity ofAviation
1.1 This guide provides information, without specific limits,
and Distillate Fuels
forselectingstandardtestmethodsfortestingaqueouspolymer
D3519 Test Method for Foam in Aqueous Media (Blender
quenchants for initial qualification, determining quality, and
Test) (Withdrawn 2013)
the effect of aging.
D3601 Test Method for Foam In Aqueous Media (Bottle
1.2 This standard does not purport to address all of the
Test) (Withdrawn 2013)
safety concerns, if any, associated with its use. It is the
D3867 Test Methods for Nitrite-Nitrate in Water
responsibility of the user of this standard to establish appro-
D4327 Test Method for Anions in Water by Suppressed Ion
priate safety, health, and environmental practices and deter-
Chromatography
mine the applicability of regulatory requirements prior to use.
D5296 Test Method for Molecular Weight Averages and
1.3 This international standard was developed in accor-
Molecular Weight Distribution of Polystyrene by High
dance with internationally recognized principles on standard-
Performance Size-Exclusion Chromatography
ization established in the Decision on Principles for the
D6482 Test Method for Determination of Cooling Charac-
Development of International Standards, Guides and Recom-
teristics of Aqueous Polymer Quenchants by Cooling
mendations issued by the World Trade Organization Technical
Curve Analysis with Agitation (Tensi Method)
Barriers to Trade (TBT) Committee.
D6549 Test Method for Determination of Cooling Charac-
teristics of Quenchants by Cooling Curve Analysis with
2. Referenced Documents
Agitation (Drayton Unit)
2.1 ASTM Standards:
E70 Test Method for pH of Aqueous Solutions With the
D95 Test Method for Water in Petroleum Products and
Glass Electrode
Bituminous Materials by Distillation
E979 Practice for Evaluation of Antimicrobial Agents as
D445 Test Method for Kinematic Viscosity of Transparent
Preservatives for Invert Emulsion and Other Water Con-
and Opaque Liquids (and Calculation of Dynamic Viscos-
taining Hydraulic Fluids
ity)
E2275 Practice for Evaluating Water-Miscible Metalwork-
D892 Test Method for Foaming Characteristics of Lubricat-
ing Fluid Bioresistance and Antimicrobial Pesticide Per-
ing Oils
formance
D1744 Test Method for Determination of Water in Liquid
Petroleum Products by Karl Fischer Reagent (Withdrawn
3. Terminology
2016)
3.1 Definitions of Terms Specific to This Standard:
D1747 Test Method for Refractive Index of Viscous Mate-
3.1.1 austenite,n—solidsolutionofoneormoreelementsin
rials
face-centered cubic iron (gamma iron) and unless otherwise
D1796 Test Method for Water and Sediment in Fuel Oils by 4
designated, the solute is generally assumed to be carbon (1).
3.1.2 austenitizing, n—forming austenite by heating a fer-
rous alloy into the transformation range (partial austenitizing)
This guide is under the jurisdiction of ASTM Committee D02 on Petroleum
Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcom- or above the transformation range (complete austenitizing).
mittee D02.L0.06 on Non-Lubricating Process Fluids.
When used without qualification, the term implies complete
CurrenteditionapprovedMay1,2019.PublishedJuly2019.Originallyapproved
austenitizing (1).
in 2001. Last previous edition approved in 2014 as D6666 – 04 (2014). DOI:
10.1520/D6666-04R19.
3.1.3 aqueous polymer quenchant, n—a solution containing
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
water, and one or more water-soluble polymers including
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
3 4
The last approved version of this historical standard is referenced on The boldface numbers in parentheses refer to the list of references at the end of
www.astm.org. this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6666 − 04 (2019)
FIG. 1 Cooling Mechanisms of the Quenching Process
poly(alkylene glycol), poly(vinyl pyrrolidone), poly(sodium 3.1.11 full-film boiling, n—upon initial immersion of hot
acrylate), and poly(ethyl oxazoline) (2, 3) and additives for steel into a quenchant solution, a vapor blanket surrounds the
corrosion and foam control, if needed. metal surface resulting in full-film boiling as shown in Fig. 1.
(5)
3.1.4 biodegradation, n—theprocessbywhichasubstrateis
converted by biological, usually microbiological, agents into 3.1.12 nucleate boiling, n—when the vapor blanket sur-
simple, environmentally acceptable derivatives. (4) rounding the hot metal collapses and a nucleate boiling process
occurs as illustrated in Fig. 1. (5)
3.1.5 biodeterioration, n—loss of product quality and per-
formance and could be regarded as the initial stages of 3.1.13 quenchant medium, n—any liquid or gas, or mixture,
biodegradation (see 3.1.4), but in the wrong place at the wrong usedtocontrolthecoolingofametaltofacilitatetheformation
time, that is when the product is stored or in use. (4) of the desired microstructure and properties. (1)
3.1.6 convective cooling, n—after continued cooling, and 3.1.14 quench severity, n—the ability of a quenchant me-
the interfacial temperature between the cooling metal and the dium to extract heat from hot metal. (6)
aqueous polymer quenchant is less than the boiling point of the
3.1.15 transformation temperatures, n—characteristic tem-
water in the quenchant solution at which point cooling occurs
peratures that are important in the formation of martensitic
by a convective cooling process. For convective cooling, fluid
microstructure of steel including: A —equilibrium austeniti-
e1
motion is due to density differences and the action of gravity
zation phase change temperature; M —temperature at which
S
and includes both natural motion and forced circulation (1, 5).
transformation of austenite to martensite starts during cooling
This process is illustrated in Fig. 1.
and M—temperature at which transformation of austenite to
f
3.1.7 cooling curve, n—a graphical representation of the martensite is completed during cooling. (1)
coolingtime(t)—temperature(T)responseoftheprobesuchas
4. Significance and Use
that shown in Fig. 1. (5)
4.1 The significance and use of each test method will
3.1.8 cooling curve analysis, n—the process of quantifying
depend on the system in use and the purpose of the test method
thecoolingcharacteristicsofaquenchantmediumbasedonthe
listed under Section 7. Use the most recent editions of the test
temperature versus time profile obtained by cooling a pre-
methods.
heated metal probe assembly (see Fig. 2) under specified
conditions which include: probe alloy and dimensions, probe
5. Quenching Process
and bath temperature, agitation rate, and aqueous polymer
5.1 Aqueous Polymer Quenchant Cooling Mechanisms—
quenchant concentration.
Upon initial immersion of a heated metal into a solution of an
3.1.9 cooling rate curve, n—obtained by calculating the first
aqueous polymer quenchant, an insulating polymer film, which
derivative (dT/dt) of the cooling time-temperature curve as
controlstheheattransferratefromthehotmetalintothecooler
illustrated in Fig. 1. (5)
quenchant solution, forms around the hot metal which is
3.1.10 dragout, n—solution carried out of a bath on the separated by a vapor film (Fig. 3) (7) for the quenching process
metal being quenched and associated handling equipment. (1) in a poly(alkylene glycol) quenchant. The overall heat transfer
D6666 − 04 (2019)
NOTE 1—From Wolfson Engineering Group Specification, available from Wolfson Heat Treatment Centre, Aston University, Aston Triangle,
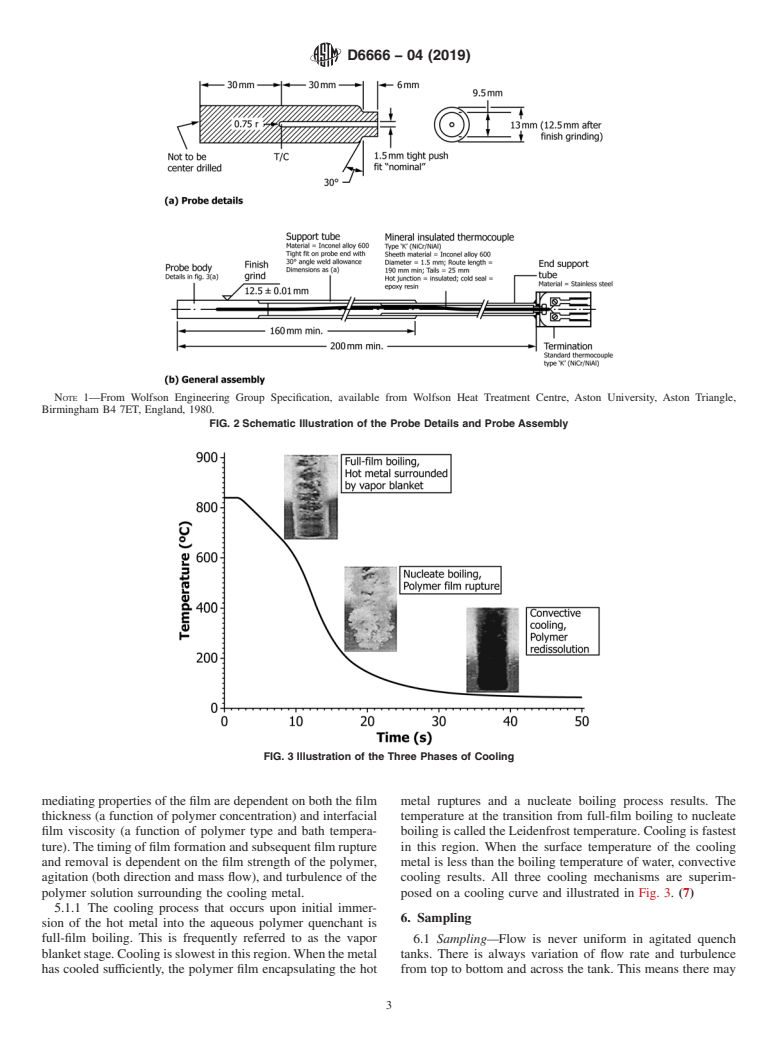
Birmingham B4 7ET, England, 1980.
FIG. 2 Schematic Illustration of the Probe Details and Probe Assembly
FIG. 3 Illustration of the Three Phases of Cooling
mediating properties of the film are dependent on both the film metal ruptures and a nucleate boiling process results. The
thickness (a function of polymer concentration) and interfacial temperature at the transition from full-film boiling to nucleate
film viscosity (a function of polymer type and bath tempera- boiling is called the Leidenfrost temperature. Cooling is fastest
ture).The timing of film formation and subsequent film rupture in this region. When the surface temperature of the cooling
and removal is dependent on the film strength of the polymer, metal is less than the boiling temperature of water, convective
agitation (both direction and mass flow), and turbulence of the cooling results. All three cooling mechanisms are superim-
polymer solution surrounding the cooling metal. posed on a cooling curve and illustrated in Fig. 3. (7)
5.1.1 The cooling process that occurs upon initial immer-
6. Sampling
sion of the hot metal into the aqueous polymer quenchant is
full-film boiling. This is frequently referred to as the vapor 6.1 Sampling—Flow is never uniform in agitated quench
blanketstage.Coolingisslowestinthisregion.Whenthemetal tanks. There is always variation of flow rate and turbulence
has cooled sufficiently, the polymer film encapsulating the hot from top to bottom and across the tank. This means there may
D6666 − 04 (2019)
(A) New aqueous polymer quenchant solution.
(B) Used quenchant solution with oil contamination (see separated upper layer).
FIG. 4 Sample of Oil Contaminated Aqueous Polymer Quenchant
be significant variations of particulate contamination including excellent test) is to examine the appearance of an aqueous
carbon from the heat treating process and metal scale. For polymer quenchant in a clear glass container, such as a bottle.
uniform sampling, a number of sampling recommendations A sample of an oil-contaminated fluid is illustrated in Fig. 4.
have been developed. (7) However, if the oil readily separates from the aqueous
6.1.1 Sampling Recommendations: polymer quenchant solution (Fig. 4), it may be removed by
6.1.1.1 Minimum Sampling Time—The circulation pumps skimming. On the other hand, oil may form a milky-white
shall be in operation for at least 1 h prior to taking a sample emulsion which is not readily reclaimed by heat treaters.
from the quench system.
7.1.1.1 Other problems that are easy to identify visually
6.1.1.2 Sampling Position—For each system, the well-
includecarbonandsludgecontaminationwhichoftenresultsin
mixed sample shall be taken from the same position each time
cracking problems. Metal scale contamination is often identi-
that system is sampled. The position in the tank where the fiable by its magnetic properties by placing a magnet on the
sample is taken shall be recorded.
outside of the bottle next to the scale and determining if the
6.1.1.3 Sampling Values—If a sample is taken from a scale exhibits any attraction for the magnet. Carbon, sludge,
sampling valve, then sufficient quenchant should be taken and
and scale may be removed from the quenchant by filtration or
discarded to ensure that the sampling valve and associated centrifugation. Alternatively, the quenchant mixture may be
piping has been flushed before the sample is taken.
allowed to settle, the quenchant solution pumped off, and the
6.1.1.4 Effect of Quenchant Addition as Make-Up due to separated solids then removed by shoveling. The amount of
Dragout—It is important to determine the quantity and fre-
insoluble suspended solids or tramp oils may be quantified by
quency of new quenchant additions, as large additions of new a modification of Test Method D1796 where the aqueous
quenchant solution will have an effect on the test results, in
quenchant is centrifuged without further dilution as described
particular, the cooling curve. If a sample was taken just after a in the method. The amount of tramp oil in the quenchant is
large addition of new quenchant, this shall be taken into
determined from the insoluble liquid layer at the top of the
consideration when interpreting the cooling curve for this centrifuge tube and the volume of the insoluble sediment is
sample.
taken from the bottom of the centrifuge tube.
6.1.1.5 Sampling Containers—Samples shall be collected in
7.1.2 Refractive Index, (Test Method D1747)—One of the
newcontainers.Undernocircumstancesshallusedbeverageor
most common methods of monitoring the concentration of
food containers be used because of the potential for fluid
aqueous polymer quenchants formulated using poly(alkylene
contamination and leakage.
glycol) coploymers is refractive index. As Fig. 5 (7) shows,
there is a linear relationship between quenchant concentration
7. Recommended Test Procedures
and refractive index. The refractive index of the quenchant
7.1 Performance-Related Physical and Chemical Proper- solution is determined using an Abbé refractometer (Test
Method D1747) equipped with a constant temperature bath.
ties:
7.1.1 Appearance—Contamination of aqueous polymer Although the refractive index could potentially be used at any
temperature within the control limits of the constant tempera-
quenchants by such fluids as hydraulic or quench oils may
ture bath, typically either 40 ºC or 100 ºF is selected.
result in a non-uniform quench with thermal gradients suffi-
cient to cause cracking or increased distortion, or possible 7.1.2.1 Although refractive index is a relatively simple and
staining,ofthemetalbeingquenched.Thesimplesttest(andan a rapid method for determination of polymer quenchant
D6666 − 04 (2019)
FIG. 5 Illustration of the Linear Relationship Between Refractive Index and Concentration
concentration, it is not sensitive to low levels of polymer ∆ 5 C 2 C (1)
R V
degradation and it is often significantly affected by solution
If the absolute value of the difference in delta is greater than
contamination.
6-8, the source of this difference, contamination or
NOTE 1—Refractive index is typically unsuitable for aqueous polymer degradation, should be determined.
quenchantsformulatedwithpolymerswithmolecularweightsgreaterthan
7.1.5 Water Content (Test Methods D95 and D1744)—
50 000 to 60 000 because the total concentration is relatively low. Small
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D6666 − 04 (Reapproved 2014) D6666 − 04 (Reapproved 2019)
Standard Guide for
Evaluation of Aqueous Polymer Quenchants
This standard is issued under the fixed designation D6666; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide provides information, without specific limits, for selecting standard test methods for testing aqueous polymer
quenchants for initial qualification, determining quality, and the effect of aging.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory requirements prior to use.
1.3 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
D95 Test Method for Water in Petroleum Products and Bituminous Materials by Distillation
D445 Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity)
D892 Test Method for Foaming Characteristics of Lubricating Oils
D1744 Test Method for Determination of Water in Liquid Petroleum Products by Karl Fischer Reagent (Withdrawn 2016)
D1747 Test Method for Refractive Index of Viscous Materials
D1796 Test Method for Water and Sediment in Fuel Oils by the Centrifuge Method (Laboratory Procedure)
D2624 Test Methods for Electrical Conductivity of Aviation and Distillate Fuels
D3519 Test Method for Foam in Aqueous Media (Blender Test) (Withdrawn 2013)
D3601 Test Method for Foam In Aqueous Media (Bottle Test) (Withdrawn 2013)
D3867 Test Methods for Nitrite-Nitrate in Water
D4327 Test Method for Anions in Water by Suppressed Ion Chromatography
D5296 Test Method for Molecular Weight Averages and Molecular Weight Distribution of Polystyrene by High Performance
Size-Exclusion Chromatography
D6482 Test Method for Determination of Cooling Characteristics of Aqueous Polymer Quenchants by Cooling Curve Analysis
with Agitation (Tensi Method)
D6549 Test Method for Determination of Cooling Characteristics of Quenchants by Cooling Curve Analysis with Agitation
(Drayton Unit)
E70 Test Method for pH of Aqueous Solutions With the Glass Electrode
E979 Practice for Evaluation of Antimicrobial Agents as Preservatives for Invert Emulsion and Other Water Containing
Hydraulic Fluids
E2275 Practice for Evaluating Water-Miscible Metalworking Fluid Bioresistance and Antimicrobial Pesticide Performance
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
This guide is under the jurisdiction of ASTM Committee D02 on Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcommittee
D02.L0.06 on Non-Lubricating Process Fluids.
Current edition approved May 1, 2014May 1, 2019. Published July 2014July 2019. Originally approved in 2001. Last previous edition approved in 20092014 as
D6666 – 04 (2009).(2014). DOI: 10.1520/D6666-04R14.10.1520/D6666-04R19.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
The last approved version of this historical standard is referenced on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6666 − 04 (2019)
FIG. 1 Cooling Mechanisms of the Quenching Process
3.1.1 austenite, n—solid solution of one or more elements in face-centered cubic iron (gamma iron) and unless otherwise
designated, the solute is generally assumed to be carbon (1).
3.1.2 austenitizing, n—forming austenite by heating a ferrous alloy into the transformation range (partial austenitizing) or above
the transformation range (complete austenitizing). When used without qualification, the term implies complete austenitizing (1).
3.1.3 aqueous polymer quenchant, n—a solution containing water, and one or more water-soluble polymers including
poly(alkylene glycol), poly(vinyl pyrrolidone), poly(sodium acrylate), and poly(ethyl oxazoline) (2, 3) and additives for corrosion
and foam control, if needed.
3.1.4 biodegradation, n—the process by which a substrate is converted by biological, usually microbiological, agents into
simple, environmentally acceptable derivatives. (4)
3.1.5 biodeterioration, n—loss of product quality and performance and could be regarded as the initial stages of biodegradation
(see 3.1.4) , ), but in the wrong place at the wrong time, that is when the product is stored or in use. (4)
3.1.6 convective cooling, n—after continued cooling, and the interfacial temperature between the cooling metal and the aqueous
polymer quenchant is less than the boiling point of the water in the quenchant solution at which point cooling occurs by a
convective cooling process. For convective cooling, fluid motion is due to density differences and the action of gravity and includes
both natural motion and forced circulation (1, 5). This process is illustrated in Fig. 1.
3.1.7 cooling curve, n—a graphical representation of the cooling time (t)—temperature (T) response of the probe such as that
shown in Fig. 1. (5)
3.1.8 cooling curve analysis, n—the process of quantifying the cooling characteristics of a quenchant medium based on the
temperature versus time profile obtained by cooling a preheated metal probe assembly (see Fig. 2) under specified conditions which
include: probe alloy and dimensions, probe and bath temperature, agitation rate, and aqueous polymer quenchant concentration.
3.1.9 cooling rate curve, n—obtained by calculating the first derivative (dT/dt) of the cooling time-temperature curve as
illustrated in Fig. 1. (5)
3.1.10 dragout, n—solution carried out of a bath on the metal being quenched and associated handling equipment. (1)
3.1.11 full-film boiling, n—upon initial immersion of hot steel into a quenchant solution, a vapor blanket surrounds the metal
surface resulting in full-film boiling as shown in Fig. 1. (5)
3.1.12 nucleate boiling, n—when the vapor blanket surrounding the hot metal collapses and a nucleate boiling process occurs
as illustrated in Fig. 1. (5)
3.1.13 quenchant medium, n—any liquid or gas, or mixture, used to control the cooling of a metal to facilitate the formation
of the desired microstructure and properties. (1)
3.1.14 quench severity, n—the ability of a quenchant medium to extract heat from hot metal. (6)
The boldface numbers in parentheses refer to the list of references at the end of this standard.
D6666 − 04 (2019)
NOTE 1—From Wolfson Engineering Group Specification, available from Wolfson Heat Treatment Centre, Aston University, Aston Triangle,
Birmingham B4 7ET, England, 1980.
FIG. 2 Schematic Illustration of the Probe Details and Probe Assembly
3.1.15 transformation temperatures, n—characteristic temperatures that are important in the formation of martensitic
microstructure of steel including: A —equilibrium austenitization phase change temperature; M —temperature at which
e1 S
transformation of austenite to martensite starts during cooling and M —temperature at which transformation of austenite to
f
martensite is completed during cooling. (1)
4. Significance and Use
4.1 The significance and use of each test method will depend on the system in use and the purpose of the test method listed under
Section 7. Use the most recent editions of the test methods.
5. Quenching Process
5.1 Aqueous Polymer Quenchant Cooling Mechanisms —Mechanisms—Upon initial immersion of a heated metal into a solution
of an aqueous polymer quenchant, an insulating polymer film, which controls the heat transfer rate from the hot metal into the
cooler quenchant solution, forms around the hot metal which is separated by a vapor film (Fig. 3) (7) for the quenching process
in a poly(alkylene glycol) quenchant. The overall heat transfer mediating properties of the film are dependent on both the film
thickness (a function of polymer concentration) and interfacial film viscosity (a function of polymer type and bath temperature).
The timing of film formation and subsequent film rupture and removal is dependent on the film strength of the polymer, agitation
(both direction and mass flow), and turbulence of the polymer solution surrounding the cooling metal.
5.1.1 The cooling process that occurs upon initial immersion of the hot metal into the aqueous polymer quenchant is full-film
boiling. This is frequently referred to as the vapor blanket stage. Cooling is slowest in this region. When the metal has cooled
sufficiently, the polymer film encapsulating the hot metal ruptures and a nucleate boiling process results. The temperature at the
transition from full-film boiling to nucleate boiling is called the Leidenfrost temperature. Cooling is fastest in this region. When
the surface temperature of the cooling metal is less than the boiling temperature of water, convective cooling results. All three
cooling mechanisms are superimposed on a cooling curve and illustrated in Fig. 3. (7)
6. Sampling
6.1 Sampling—Flow is never uniform in agitated quench tanks. There is always variation of flow rate and turbulence from top
to bottom and across the tank. This means there may be significant variations of particulate contamination including carbon from
the heat treating process and metal scale. For uniform sampling, a number of sampling recommendations have been developed.
6.1.1 Sampling Recommendations:
6.1.1.1 Minimum Sampling Time—The circulation pumps shall be in operation for at least 1 h 1 h prior to taking a sample from
the quench system.
6.1.1.2 Sampling Position—For each system, the well-mixed sample shall be taken from the same position each time that system
is sampled. The position in the tank where the sample is taken shall be recorded.
D6666 − 04 (2019)
FIG. 3 Illustration of the Three Phases of Cooling
6.1.1.3 Sampling Values—If a sample is taken from a sampling valve, then sufficient quenchant should be taken and discarded
to ensure that the sampling valve and associated piping has been flushed before the sample is taken.
6.1.1.4 Effect of Quenchant Addition as Make-Up due to Dragout—It is important to determine the quantity and frequency of
new quenchant additions, as large additions of new quenchant solution will have an effect on the test results, in particular, the
cooling curve. If a sample was taken just after a large addition of new quenchant, this shall be taken into consideration when
interpreting the cooling curve for this sample.
6.1.1.5 Sampling Containers—Samples shall be collected in new containers. Under no circumstances shall used beverage or
food containers be used because of the potential for fluid contamination and leakage.
7. Recommended Test Procedures
7.1 Performance-Related Physical and Chemical Properties:
7.1.1 Appearance—Contamination of aqueous polymer quenchants by such fluids as hydraulic or quench oils may result in a
non-uniform quench with thermal gradients sufficient to cause cracking or increased distortion, or possible staining, of the metal
being quenched. The simplest test (and an excellent test) is to examine the appearance of an aqueous polymer quenchant in a clear
glass container, such as a bottle. A sample of an oil-contaminated fluid is illustrated in Fig. 4. (7) However, if the oil readily
separates from the aqueous polymer quenchant solution (Fig. 4), it may be removed by skimming. On the other hand, oil may form
a milky-white emulsion which is not readily reclaimed by heat treaters.
7.1.1.1 Other problems that are easy to identify visually include carbon and sludge contamination which often results in
cracking problems. Metal scale contamination is often identifiable by its magnetic properties by placing a magnet on the outside
of the bottle next to the scale and determining if the scale exhibits any attraction for the magnet. Carbon, sludge, and scale may
be removed from the quenchant by filtration or centrifugation. Alternatively, the quenchant mixture may be allowed to settle, the
quenchant solution pumped off, and the separated solids then removed by shoveling. The amount of insoluble suspended solids
or tramp oils may be quantified by a modification of Test Method D1796 where the aqueous quenchant is centrifuged without
further dilution as described in the method. The amount of tramp oil in the quenchant is determined from the insoluble liquid layer
at the top of the centrifuge tube and the volume of the insoluble sediment is taken from the bottom of the centrifuge tube.
7.1.2 Refractive Index, (Test Method D1747)—One of the most common methods of monitoring the concentration of aqueous
polymer quenchants formulated using poly(alkylene glycol) coploymers is refractive index. As Fig. 5 (7) shows, there is a linear
relationship between quenchant concentration and refractive index. The refractive index of the quenchant solution is determined
using an Abbé refractometer (Test Method D1747) equipped with a constant temperature bath. Although the refractive index could
potentially be used at any temperature within the control limits of the constant temperature bath, typically either 40ºC40 ºC or
100ºF100 ºF is selected.
7.1.2.1 Although refractive index is a relatively simple and a rapid method for determination of polymer quenchant
concentration, it is not sensitive to low levels of polymer degradation and it is often significantly affected by solution
contamination.
NOTE 1—Refractive index is typically unsuitable for aqueous polymer quenchants formulated with polymers with molecular weights greater than 50
000 to 60 000 because the total concentration is relatively low. Small changes in polymer concentration may result even from normal use which impart
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.