ASTM F702-10
(Specification)Standard Specification for Polysulfone Resin for Medical Applications
Standard Specification for Polysulfone Resin for Medical Applications
ABSTRACT
This specification covers polysulfone resin (poly(oxy-p-phenylenesulfonyl-p-phenyleneoxy-p-phenyleneisopropylidene-p-phenylene)) for medical applications. Requirements and associated test methods for a form of this thermoplastic intended for use in manufacturing medical devices or components of medical devices are provided. The use of this resin in medical devices should be restricted to nonimplant applications until biocompatibility evaluations appropriate for the intended applications are successfully completed. The molecular weight of the resin shall be determined by osmotic pressure in monochlorobenzene. The polysulfone resin shall yield an infrared transmittance spectrum that exhibits major transmittance bands only at the same wavelengths as that of a reference spectrum. Medical devices made of polysulfone may be repeatedly sterilized through steam, ethylene oxide, irradiation, and dry heat sterilization, among others. The polysulfone resin shall be tested for nonvolatile content and melt flow, and shall conform to the specified electrical, physical and mechanical, and thermal properties.
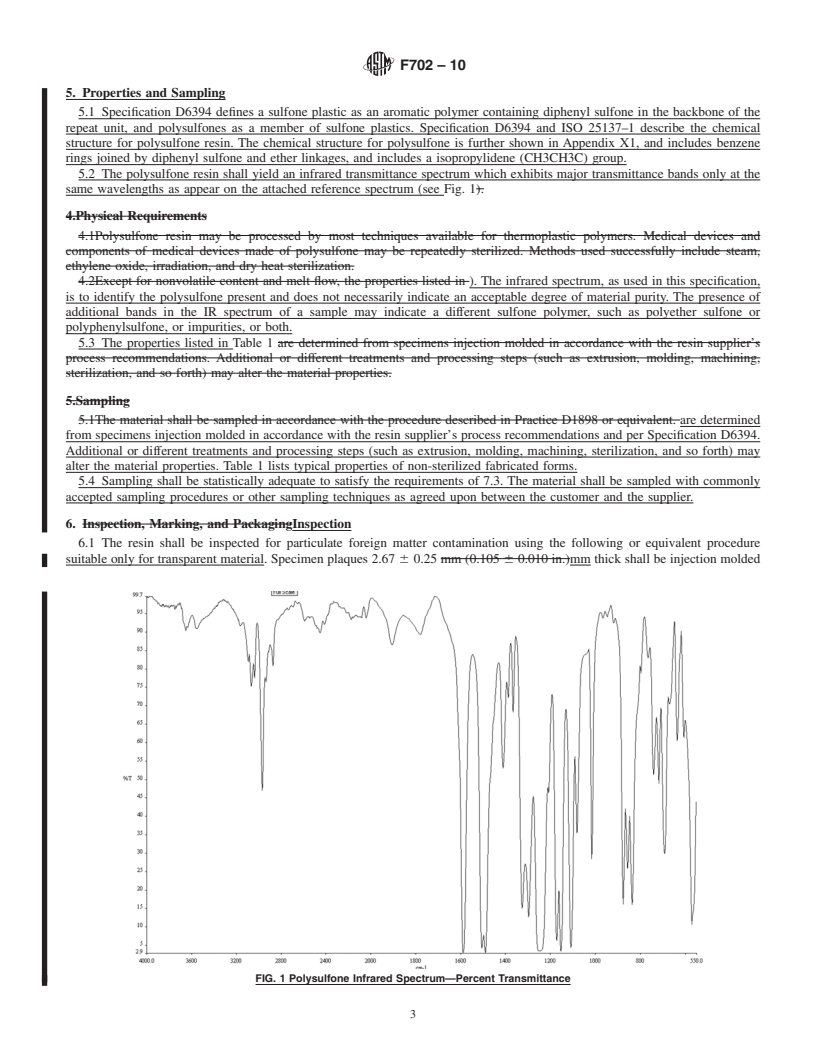
SCOPE
1.1 This specification covers polysulfone resin (poly(oxy-1,4-phenylenesulfonyl-1,4–phenylene (dimethylmethylene)-1,4–phenylene)) as defined in ISO 25137–1, supplied by a vendor in virgin form (pellets, powder, fabricated forms and so forth) for medical applications. This specification provides requirements and associated test methods for this thermoplastic when it is intended for use in manufacturing medical devices or components of medical devices.
1.2 As with any material, some characteristics may be altered by the processing techniques (such as molding, extrusion, machining, sterilization, and so forth) required for the production of a specific part or device. Therefore, properties of fabricated forms of this resin should be evaluated using test methods which are appropriate to ensure safety and efficacy as agreed upon by the vendor, purchaser, and regulating bodies.
1.3 The standard allows for designation of polysulfone resin for all medical applications. The actual extent of performance and suitability for a specific application must be evaluated by the vendor, purchaser, and regulating bodies.
1.4 The properties included in this specification are those applicable for unfilled polysulfone (PSU) polymers with the addition of colorants and processing aids. Indicated properties are for injection molded forms. Forms containing fillers or other additives, as well as polymer blends which contain PSU, or reclaimed materials, are not covered by this specification.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 When evaluating material in accordance with this specification, hazardous materials, operations, and equipment may be involved. This standard does not purport to address all of the concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F702 −10
Standard Specification for
1
Polysulfone Resin for Medical Applications
ThisstandardisissuedunderthefixeddesignationF702;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
1.1 This specification covers polysulfone resin (poly(oxy-1,
4-phenylenesulfonyl-1,4–phenylene (dimethylmethylene)-1,
2. Referenced Documents
4–phenylene)) as defined in ISO 25137–1, supplied by a
2
vendorinvirginform(pellets,powder,fabricatedformsandso
2.1 ASTM Standards:
forth) for medical applications. This specification provides
D256Test Methods for Determining the Izod Pendulum
requirementsandassociatedtestmethodsforthisthermoplastic
Impact Resistance of Plastics
whenitisintendedforuseinmanufacturingmedicaldevicesor
D638Test Method for Tensile Properties of Plastics
components of medical devices.
D648Test Method for Deflection Temperature of Plastics
Under Flexural Load in the Edgewise Position
1.2 As with any material, some characteristics may be
D792Test Methods for Density and Specific Gravity (Rela-
altered by the processing techniques (such as molding,
tive Density) of Plastics by Displacement
extrusion, machining, sterilization, and so forth) required for
D6394Specification for Sulfone Plastics (SP)
the production of a specific part or device. Therefore, proper-
F748PracticeforSelectingGenericBiologicalTestMethods
ties of fabricated forms of this resin should be evaluated using
for Materials and Devices
test methods which are appropriate to ensure safety and
3
efficacy as agreed upon by the vendor, purchaser, and regulat-
2.2 ISO Standards:
ing bodies.
ISO 10993Biological Evaluation of Medical Devices
ISO 17025General Requirements for the Competence of
1.3 Thestandardallowsfordesignationofpolysulfoneresin
Testing and Calibration Laboratories
for all medical applications. The actual extent of performance
ISO 25137–1Plastics—Sulfone Polymer Moulding and Ex-
and suitability for a specific application must be evaluated by
trusion Materials—Part I: Designation System and Basis
the vendor, purchaser, and regulating bodies.
for Specifications
1.4 The properties included in this specification are those
applicable for unfilled polysulfone (PSU) polymers with the
3. Significance and Use
addition of colorants and processing aids. Indicated properties
are for injection molded forms. Forms containing fillers or
3.1 This specification is designed to recommend test meth-
other additives, as well as polymer blends which contain PSU,
odstoestablishareasonablelevelofconfidenceconcerningthe
or reclaimed materials, are not covered by this specification.
performance of unfilled polysulfone resins for use in medical
devices.Thepropertieslistedshouldbeconsideredinselecting
1.5 The values stated in SI units are to be regarded as
material according to specific end-use requirements.
standard. No other units of measurement are included in this
standard.
3.2 Polysulfones may be evaluated in implantable medical
1.6 When evaluating material in accordance with this devices as well as in non-implant medical applications. Poly-
specification, hazardous materials, operations, and equipment sulfone resins intended for use in implant applications are
maybeinvolved. This standard does not purport to address all manufactured with more rigorous use of manufacturing and/or
of the concerns, if any, associated with its use. It is the testingcontrols,toassureconsistencyofproperties,cleanliness
responsibility of the user of this standard to establish appro- and biocompatibility. This is further elaborated in 4.1.
1 2
This specification is under the jurisdiction of ASTM Committee F04 on For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Medical and Surgical Materials and Devices and is the direct responsibility of contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Subcommittee F04.11 on Polymeric Materials. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Sept. 1, 2010. Published October 2010. Originally the ASTM website.
3
approved in 1981. Last previous edition approved in 2003 as F702–98a (2003). Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
DOI: 10.1520/F0702-10. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F702−10
FIG. 1 Polysulfone Infrared Spectrum—Percent Transmittanc
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:F702–98a (Reapproved 2003) Designation: F702 – 10
Standard Specification for
1
Polysulfone Resin for Medical Applications
ThisstandardisissuedunderthefixeddesignationF702;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1This specification covers polysulfone resin (poly(oxy-p-phenylenesulfonyl- p-phenyleneoxy-p-phenyleneisopropylidene-p-
phenylene)) for medical applications (as defined in Terminology D883). This specification provides requirements and associated
testmethodsforaformofthisthermoplasticwhichisintendedforuseinmanufacturingmedicaldevicesorcomponentsofmedical
devices.
1.2As with any material, some characteristics may be altered by the processing techniques (such as molding, extrusion,
machining, sterilization, and so forth) required for a specific application. Therefore, properties of fabricated forms of this resin
should be evaluated using appropriate test methods to assure safety and efficacy.
1.3The use of this resin in medical devices should be restricted to nonimplant applications until biocompatibility evaluations
appropriate for the intended applications are successfully completed.
1.4The biocompatibility of plastic compounds made up of polysulfone resin containing colorants, fillers, processing aids, or
other additives as well as polymer blends which contain polysulfone should not be assumed on the basis of resin compatibility
alone.Their biocompatibility must be established by testing the final (end-use) compositions using evaluation methods appropriate
for the intended applications. Note that the types, levels, and biological effects of extractives yielded by the additives contained
in a compound or blend may also have to be evaluated for some end-use applications.
1.5All values in this standard are in SI units with the equivalent values in inch-pound units given in parentheses where
applicable.
1.6
1.1 This specification covers polysulfone resin (poly(oxy-1,4-phenylenesulfonyl-1,4–phenylene (dimethylmethylene)-
1,4–phenylene)) as defined in ISO 25137–1, supplied by a vendor in virgin form (pellets, powder, fabricated forms and so forth)
for medical applications. This specification provides requirements and associated test methods for this thermoplastic when it is
intended for use in manufacturing medical devices or components of medical devices.
1.2 As with any material, some characteristics may be altered by the processing techniques (such as molding, extrusion,
machining, sterilization, and so forth) required for the production of a specific part or device. Therefore, properties of fabricated
forms of this resin should be evaluated using test methods which are appropriate to ensure safety and efficacy as agreed upon by
the vendor, purchaser, and regulating bodies.
1.3 The standard allows for designation of polysulfone resin for all medical applications. The actual extent of performance and
suitability for a specific application must be evaluated by the vendor, purchaser, and regulating bodies.
1.4 The properties included in this specification are those applicable for unfilled polysulfone (PSU) polymers with the addition
of colorants and processing aids. Indicated properties are for injection molded forms. Forms containing fillers or other additives,
as well as polymer blends which contain PSU, or reclaimed materials, are not covered by this specification.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 When evaluating material in accordance with this specification, hazardous materials, operations, and equipment may be
involved. This standard does not purport to address all of the concerns, if any, associated with its use. It is the responsibility of
the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
1
This specification is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.11 on Polymeric Materials.
´1
Current edition approved Apr. 10, 2003. Published May 2003. Originally approved in 1981. Last previous edition approved in 1998 as F702–98a . DOI:
10.1520/F0702-98AR03.
Current edition approved Sept. 1, 2010. Published October 2010. Originally
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.