ASTM F2182-02a
(Test Method)Standard Test Method for Measurement of Radio Frequency Induced Heating Near Passive Implants During Magnetic Resonance Imaging
Standard Test Method for Measurement of Radio Frequency Induced Heating Near Passive Implants During Magnetic Resonance Imaging
SIGNIFICANCE AND USE
This test method describes a test procedure for evaluating the RF-induced temperature rise in MRI in the vicinity of an implanted medical device. The actual temperature rise in the patient will depend on a variety of factors beyond the SAR and time of RF application. The conditions and results of the testing should be included in the device labeling so that the attending physician can make the decision of whether to allow the patient with the implant to undergo an MRI procedure.
SCOPE
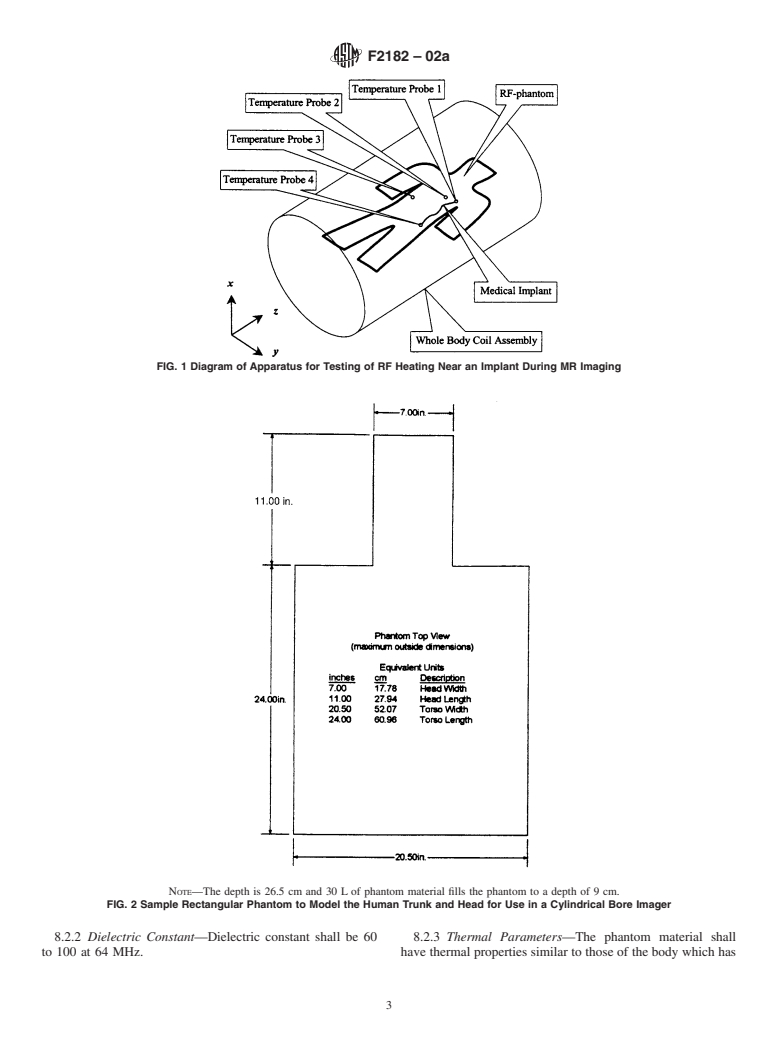
1.1 This test method covers measurement of Radio Frequency (RF) induced heating near a passive medical implant and its surroundings during Magnetic Resonance Imaging (MRI).
1.2 This test method is one of those required to determine if the presence of a passive implant may cause injury to the person with the implant during an MRI procedure. Other safety issues that should be addressed include magnetically induced displacement force and torque.
1.3 The amount of RF-induced temperature rise for a given specific absorption rate (SAR) will depend on the RF frequency, which is proportional to the static magnetic field strength. Because of possible additional heating, particularly when device dimensions exceed a quarter wavelength, conclusions from measurements made at one frequency may not apply to other frequencies.
1.4 This test method assumes that testing is done on devices that will be entirely inside the body.
1.5 This test method applies to whole body magnetic resonance equipment, as defined in section 2.2.103 of the IEC Standard 60601-2-33 with a whole body RF transmit coil as defined in section 2.2.100. The RF coil is assumed to have quadrature excitation.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information.
Designation:F2182–02a
Standard Test Method for
Measurement of Radio Frequency Induced Heating Near
1
Passive Implants During Magnetic Resonance Imaging
This standard is issued under the fixed designation F2182; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
1.1 This test method covers measurement of Radio Fre- 2.1 ASTM Standards:
quency (RF) induced heating near a passive medical implant A340 Terminology of Symbols and Definitions Relating to
3
and its surroundings during Magnetic Resonance Imaging Magnetic Testing
(MRI). F2052 Test Method for Measurement of Magnetically In-
1.2 This test method is one of those required to determine if duced Displacement Force on Medical Devices in the
3
the presence of a passive implant may cause injury to the Magnetic Resonance Environment
personwiththeimplantduringanMRIprocedure.Othersafety F2119 Test Method for Evaluation of MR Image Artifacts
issues that should be addressed include magnetically induced from Passive Implants
4
displacement force and torque. 2.2 IEC Standard:
1.3 The amount of RF-induced temperature rise for a given 60601-2-33, Ed. 2.0 Medical Electrical Equipment—Part 2:
specific absorption rate (SAR) will depend on the RF fre- Particular Requirements for the Safety of Magnetic Reso-
quency, which is proportional to the static magnetic field nance Equipment for Medical Diagnosis, 2002
strength. Because of possible additional heating, particularly
3. Terminology
when device dimensions exceed a quarter wavelength, conclu-
sions from measurements made at one frequency may not 3.1 Definitions—For the purposes of this test method, the
definitions in 3.1.1-3.1.10 shall apply.
apply to other frequencies. While the focus in this test method
is on 1.5 T cylindrical bore imagers, the RF-induced tempera- 3.1.1 isocenter—geometric center of the gradient coil sys-
tem, which generally is the geometric center of a scanner with
ture rise in the open MRI systems can be evaluated by suitable
a cylindrical bore.
modification of the methods described here.
1.4 This test method assumes that testing is done on devices 3.1.2 magnetic resonance imaging (MRI)—diagnostic im-
aging technique that uses static and time varying magnetic
that will be entirely inside the body.
1.5 This test method applies to whole body magnetic fields to provide images of tissue by the magnetic resonance of
nuclei.
resonance equipment, as defined in section 2.2.103 of the IEC
Standard 60601-2-33, Ed. 2.0, with a whole body RF transmit 3.1.3 magnetic resonance (MR) environment—area within
the 5 G line of an MR system.
coil as defined in section 2.2.100. The RF coil is assumed to
have quadrature excitation. 3.1.4 magnetic resonance system (MR System)—ensemble
of MR equipment, accessories including means for display,
1.6 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the control, energy supplies, and the MR environment.
3.1.5 medical implant—a structure or device that is placed
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- within the body of the patient for medical diagnostic or
therapeutic purposes.
bility of regulatory limitations prior to use.
3.1.6 MR safe—the device, when used in the MR environ-
ment, has been demonstrated to present no additional risk to
1
This test method is under the jurisdiction ofASTM Committee F04 on Medical
andSurgicalMaterialsandDevices andisthedirectresponsibilityofSubcommittee
2
F04.15 on Material Test Methods. Annual Book of ASTM Standards, Vol 03.04.
3
Current edition approved Nov. 10, 2002. Published December 2002. Originally Annual Book of ASTM Standards, Vol 13.01.
4
approved in 2002. Last previous edition approved in 2002 as F2182 – 02. DOI: Available from the International Electrotechnical Commission (IEC), 3 rue de
10.1520/F2182-02A. Varembe, Case postale 131, CH-1211 Geneva 20, Switzerland.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
F2182–02a
the patient or other individuals, but may affect the quality of 6.2 Temperature Sensor—Asuitable temperature measuring
the diagnostic information. The MR conditions in which the device, usually a fiber optic probe, is used to measure tempera-
device was tested should be specified in conjunction with the ture versus time of RF exposure in the vicinity of the implant.
termsMRsafeandMRcompatiblesinceadevicewhichissafe The
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.