ASTM F2077-11
(Test Method)Test Methods For Intervertebral Body Fusion Devices
Test Methods For Intervertebral Body Fusion Devices
SIGNIFICANCE AND USE
Intervertebral body fusion device assemblies are generally simple geometric-shaped devices which are often porous or hollow in nature. Their function is to support the anterior column of the spine to facilitate arthrodesis of the motion segment. This test method outlines materials and methods for the characterization and evaluation of the mechanical performance of different intervertebral body fusion device assemblies so that comparisons can be made between different designs.
This test method is designed to quantify the static and dynamic characteristics of different designs of intervertebral body fusion device assemblies. These tests are conducted in vitro to allow for analysis and comparison of the mechanical performance of intervertebral body fusion device assemblies to specific force modalities.
The forces applied to the intervertebral body fusion assemblies may differ from the complex loading seen in vivo, and therefore, the results from these tests may not directly predict in vivo performance. The results, however, can be used to compare mechanical performance of different intervertebral body fusion device assemblies.
Since the environment may affect the dynamic performance of intervertebral body fusion device assemblies, dynamic testing in a saline environment may be considered. Fatigue tests should first be conducted in air (at ambient temperature) for comparison purposes since the environmental effects could be significant. If a simulated in vivo environment is desired, the investigator should consider testing in a saline environmental bath at 37°C (for example, 0.9-g NaCl per 100-mL water) at a rate of 1 Hz or less. A simulated body fluid, a saline drip or mist, distilled water, or other type of lubrication at 37°C could also be used with adequate justification.
If the devices are known to be temperature and environment dependent, testing should be conducted in physiologic solution as described in 5.4. Devices that require physiologic solution for ...
SCOPE
1.1 This test method covers the materials and methods for the static and dynamic testing of intervertebral body fusion device assemblies, spinal implants designed to promote arthrodesis at a given spinal motion segment.
1.2 This test method is intended to provide a basis for the mechanical comparison among past, present, and future nonbiologic intervertebral body fusion device assemblies. This test method allows comparison of intervertebral body fusion device assemblies with different intended spinal locations and methods of application to the intradiscal spaces. This test method is intended to enable the user to compare intervertebral body fusion device assemblies mechanically and does not purport to provide performance standards for intervertebral body fusion device assemblies.
1.3 The test method describes static and dynamic tests by specifying force types and specific methods of applying these forces. These tests are designed to allow for the comparative evaluation of intervertebral body fusion device assemblies.
1.4 These tests are designed to characterize the structural integrity of the device and are not intended to test the bone-implant interface.
1.5 This test method does not address expulsion testing of intervertebral body fusion device assemblies (see 1.4).
1.6 Guidelines are established for measuring displacements, determining the yield force or moment, evaluating the stiffness, and strength of the intervertebral body fusion device assemblies.
1.7 Some intervertebral body fusion device assemblies may not be testable in all test configurations.
1.8 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard, with the exception of angular measurements, which may be reported in terms of either degrees or radians.
1.9 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the respon...
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2077 − 11
TestMethods For
1
Intervertebral Body Fusion Devices
This standard is issued under the fixed designation F2077; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.9 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
1.1 This test method covers the materials and methods for
responsibility of the user of this standard to establish appro-
the static and dynamic testing of intervertebral body fusion
priate safety and health practices and determine the applica-
device assemblies, spinal implants designed to promote arthro-
bility of regulatory limitations prior to use.
desis at a given spinal motion segment.
1.2 This test method is intended to provide a basis for the
2. Referenced Documents
mechanical comparison among past, present, and future non-
2
2.1 ASTM Standards:
biologic intervertebral body fusion device assemblies.This test
E4 Practices for Force Verification of Testing Machines
methodallowscomparisonofintervertebralbodyfusiondevice
E6 Terminology Relating to Methods of Mechanical Testing
assemblies with different intended spinal locations and meth-
E1823 TerminologyRelatingtoFatigueandFractureTesting
ods of application to the intradiscal spaces. This test method is
E2309 Practices for Verification of Displacement Measuring
intended to enable the user to compare intervertebral body
Systems and Devices Used in Material Testing Machines
fusion device assemblies mechanically and does not purport to
F1582 Terminology Relating to Spinal Implants
provide performance standards for intervertebral body fusion
device assemblies.
3. Terminology
1.3 The test method describes static and dynamic tests by
3.1 For definition of terms refer to Terminology E6, E1823,
specifying force types and specific methods of applying these
and F1582.
forces. These tests are designed to allow for the comparative
evaluation of intervertebral body fusion device assemblies. 3.2 Definitions of Terms Specific to This Standard:
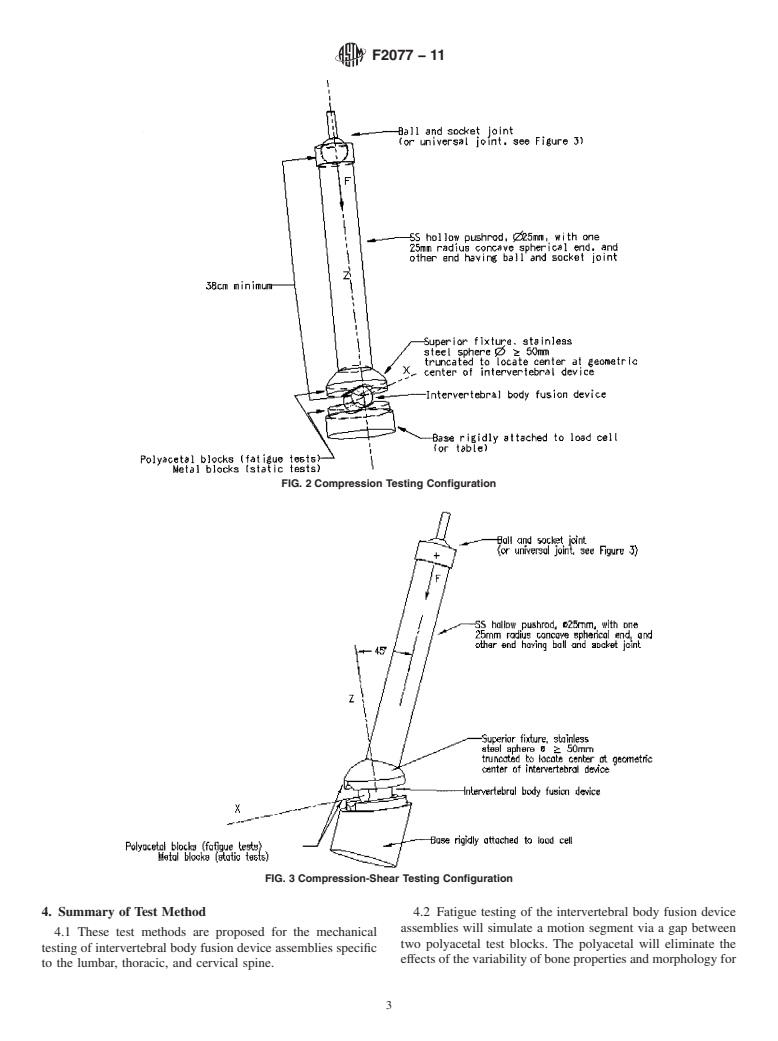
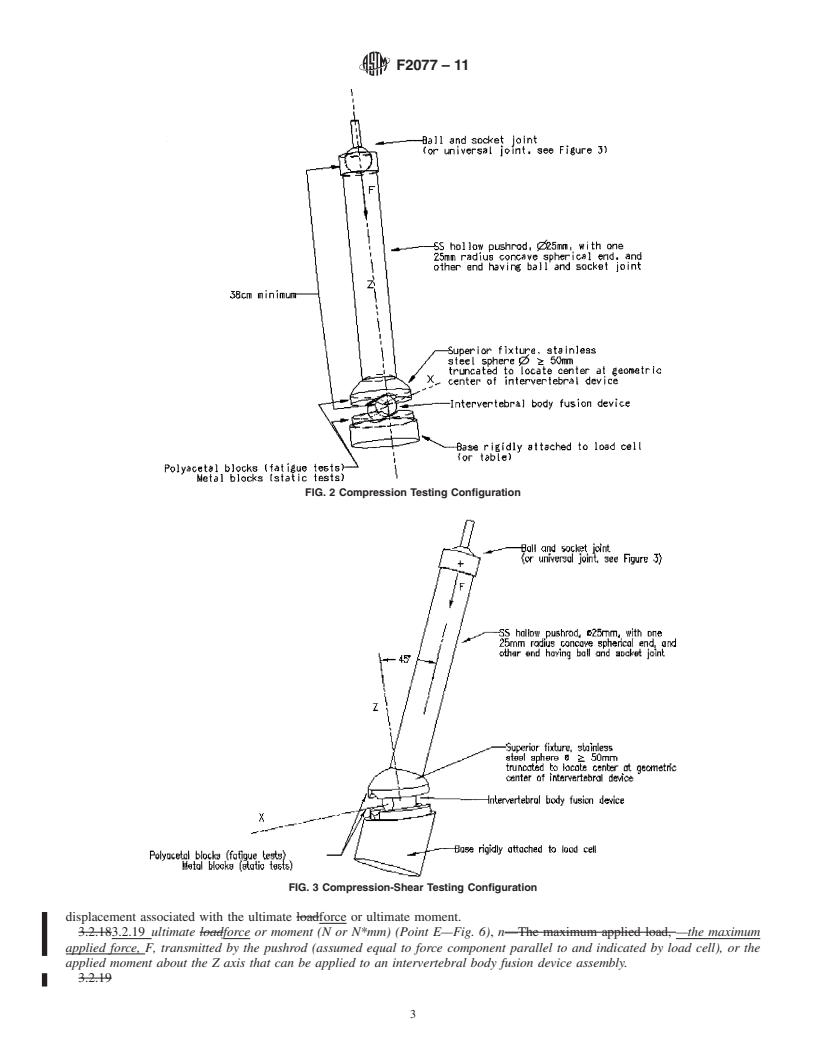
3.2.1 coordinate system/axes, n—Three orthogonal axes are
1.4 These tests are designed to characterize the structural
defined by Terminology F1582. The center of the coordinate
integrity of the device and are not intended to test the
system is located at the geometric center of the intervertebral
bone-implant interface.
body fusion device assembly. The XY plane is to bisect the
1.5 This test method does not address expulsion testing of
sagittal plane angle between superior and inferior lines (sur-
intervertebral body fusion device assemblies (see 1.4).
faces) that are intended to simulate the adjacent vertebral end
plates. The positive Z axis is to be directed superiorly. Force
1.6 Guidelines are established for measuring displacements,
components parallel to the XY plane are shear components of
determiningtheyieldforceormoment,evaluatingthestiffness,
loading. The compressive axial force is defined to be the
and strength of the intervertebral body fusion device assem-
component in the negative Z direction. Torsional force is
blies.
defined to be the component of moment parallel to the Z axis.
1.7 Some intervertebral body fusion device assemblies may
3.2.2 crack, n—an externally visible physical discontinuity
not be testable in all test configurations.
in the form of a narrow opening that arises from mechanical
1.8 The values stated in SI units are to be regarded as
forces.
standard. No other units of measurement are included in this
3.2.3 fatigue life, n—the number of cycles, N, that the
standard, with the exception of angular measurements, which
intervertebral body fusion device assembly can sustain at a
may be reported in terms of either degrees or radians.
particular force or moment before mechanical or functional
failure occurs.
1
This test method is under the jurisdiction ofASTM Committee F04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
2
F04.25 . For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved July 15, 2011. Published August 2011. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
published in 2000. Last previous edition approved in 2003 as F2077 - 03. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F2077-11. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2077 − 11
3.2.4 functional failure, n—perman
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:F2077–03 Designation: F2077 – 11
Test Methods For
1
Intervertebral Body Fusion Devices
This standard is issued under the fixed designation F2077; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the materials and methods for the static and dynamic testing of intervertebral body fusion device
assemblies, spinal implants designed to promote arthrodesis at a given spinal motion segment.
1.2 This test method is intended to provide a basis for the mechanical comparison among past, present, and future nonbiologic
intervertebral body fusion device assemblies. This test method allows comparison of intervertebral body fusion device assemblies
with different intended spinal locations and methods of application to the intradiscal spaces.This test method is intended to enable
the user to compare intervertebral body fusion device assemblies mechanically and does not purport to provide performance
standards for intervertebral body fusion device assemblies.
1.3The test method describes static and dynamic tests by specifying load types and specific methods of applying these loads.
These tests are designed to allow for the comparative evaluation of intervertebral body fusion device assemblies.
1.4This test method does not address expulsion testing of intervertebral body fusion device assemblies (see X1.11
1.3 The test method describes static and dynamic tests by specifying force types and specific methods of applying these forces.
These tests are designed to allow for the comparative evaluation of intervertebral body fusion device assemblies.
1.4 These tests are designed to characterize the structural integrity of the device and are not intended to test the bone-implant
interface.
1.5 This test method does not address expulsion testing of intervertebral body fusion device assemblies (see 1.4).
1.56 Guidelines are established for measuring displacements, determining the yield loadforce or moment, evaluating the
stiffness, and strength of the intervertebral body fusion device assemblies.
1.6Some1.7 Some intervertebral body fusion device assemblies may not be testable in all test configurations.
1.7The1.8 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this
standard, with the exception of angular measurements, which may be reported in terms of either degrees or radians.
1.81.9 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
E4 Practices for Force Verification of Testing Machines
E6 Terminology Relating to Methods of Mechanical Testing
E1823 Terminology Relating to Fatigue and Fracture Testing
E2309 Practices for Verification of Displacement Measuring Systems and Devices Used in Material Testing Machines
F1582 Terminology Relating to Spinal Implants
3. Terminology
3.1 For definition of terms refer to Terminology E6, E1823, and F1582.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 coordinatesystem/axes,n—ThreeorthogonalaxesaredefinedbyTerminologyF1582.Thecenterofthecoordinatesystem
is located at the geometric center of the intervertebral body fusion device assembly. The XY plane is to bisect the sagittal plane
angle between superior and inferior lines (surfaces) that are intended to simulate the adjacent vertebral end plates. The positive
1
This test method is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.25 on Spinal Devices .
Current edition approvedApr. 10, 2003. Published May 2003. Originally published in 2000. Last previous edition approved in 2001 as F2077-01. DOI: 10.1520/F2077-03.
Current edition approved July 15, 2011. Published August 2011. Originally published in 2000. Last previous edition approved in 2003 as F2077 - 03. DOI:
10.1520/F2077-11.
2
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’’s Document Summary
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.