ASTM F1875-98(2014)
(Practice)Standard Practice for Fretting Corrosion Testing of Modular Implant Interfaces: Hip Femoral Head-Bore and Cone Taper Interface
Standard Practice for Fretting Corrosion Testing of Modular Implant Interfaces: Hip Femoral Head-Bore and Cone Taper Interface
SIGNIFICANCE AND USE
5.1 The modular interfaces of total joint prostheses are subjected to micromotion that could result in fretting and corrosion. The release of corrosion products and particulate debris could stimulate adverse biological reactions, as well as lead to accelerated wear at the articulation interface. Methods to assess the stability and corrosion resistance of the modular interfaces, therefore, are an essential component of device testing.
5.2 Long-term in-vitro testing is essential to produce damage and debris from fretting of a modular interface (4,5). The use of proteinaceous solutions is recommended to best simulate the in-vivo environment.
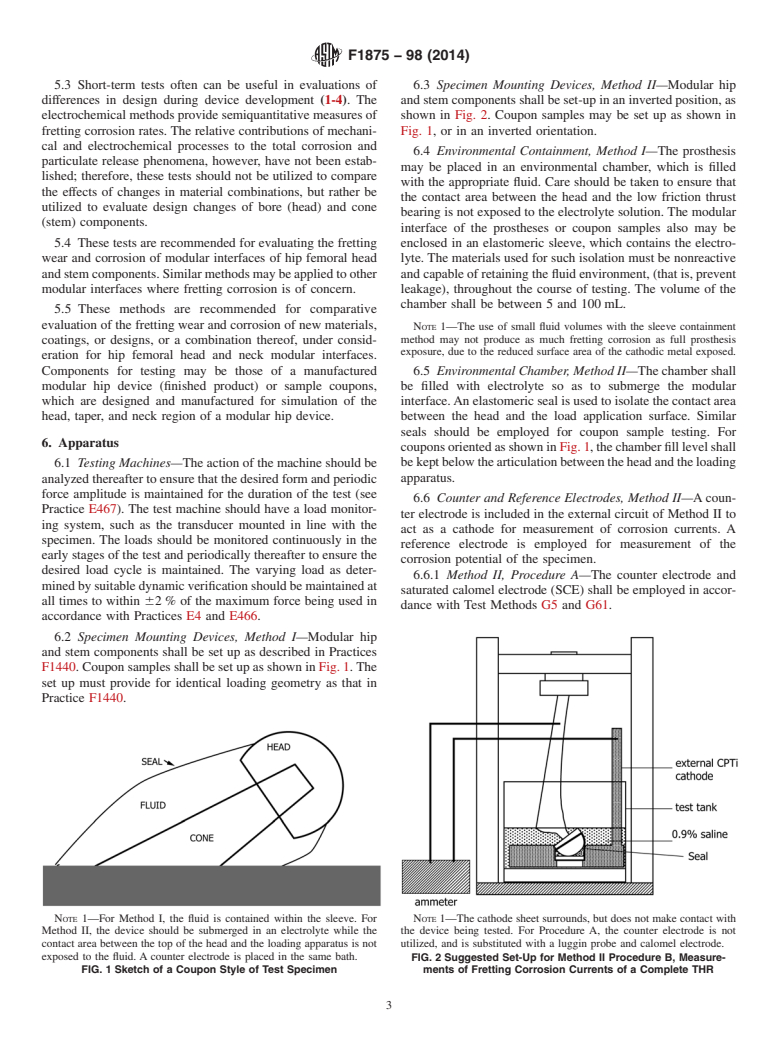
5.3 Short-term tests often can be useful in evaluations of differences in design during device development (1-4). The electrochemical methods provide semiquantitative measures of fretting corrosion rates. The relative contributions of mechanical and electrochemical processes to the total corrosion and particulate release phenomena, however, have not been established; therefore, these tests should not be utilized to compare the effects of changes in material combinations, but rather be utilized to evaluate design changes of bore (head) and cone (stem) components.
5.4 These tests are recommended for evaluating the fretting wear and corrosion of modular interfaces of hip femoral head and stem components. Similar methods may be applied to other modular interfaces where fretting corrosion is of concern.
5.5 These methods are recommended for comparative evaluation of the fretting wear and corrosion of new materials, coatings, or designs, or a combination thereof, under consideration for hip femoral head and neck modular interfaces. Components for testing may be those of a manufactured modular hip device (finished product) or sample coupons, which are designed and manufactured for simulation of the head, taper, and neck region of a modular hip device.
SCOPE
1.1 This practice describes the testing, analytical, and characterization methods for evaluating the mechanical stability of the bore and cone interface of the head and stem junction of modular hip implants subjected to cyclic loading by measurements of fretting corrosion (1-5).2 Two test methods described are as follows:
1.1.1 Method I—The primary purpose of this method is to provide a uniform set of guidelines for long-term testing to determine the amount of damage by measurement of the production of corrosion products and particulate debris from fretting and fretting corrosion. Damage is also assessed by characterization of the damage to the bore and cone surfaces (4, 5).
1.1.2 Methods II—This method provides for short-term electrochemical evaluation of the fretting corrosion of the modular interface. It is not the intent of this method to produce damage nor particulate debris but rather to provide a rapid method for qualitative assessment of design changes which do not include material changes (1-4).
1.2 This practice does not provide for judgment or prediction of in-vivo implant performance, but rather provides for a uniform set of guidelines for evaluating relative differences in performance between differing implant designs, constructs, or materials with performance defined in the context of the amount of fretting and fretting corrosion. Also, this practice should permit direct comparison of fretting corrosion data between independent research groups, and thus provide for building of a data base on modular implant performance.
1.3 This practice provides for comparative testing of manufactured hip femoral heads and stems and for coupon type specimen testing where the male taper portion of the modular junction does not include the entire hip implant, with the taper portion of the coupon identical in design, manufacturing, and materials to the taper of the final hip implant (4,5).
1.4 Method I of this practice permits simultaneous evaluation of the fatigue strength of a femoral h...
General Information
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F1875 − 98 (Reapproved 2014)
Standard Practice for
Fretting Corrosion Testing of Modular Implant Interfaces:
1
Hip Femoral Head-Bore and Cone Taper Interface
This standard is issued under the fixed designation F1875; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope portion of the coupon identical in design, manufacturing, and
materials to the taper of the final hip implant (4,5).
1.1 This practice describes the testing, analytical, and char-
acterization methods for evaluating the mechanical stability of
1.4 Method I of this practice permits simultaneous evalua-
the bore and cone interface of the head and stem junction of
tionofthefatiguestrengthofafemoralhipstem(inaccordance
modular hip implants subjected to cyclic loading by measure-
with Practice F1440) and the mechanical stability and debris
2
ments of fretting corrosion (1-5). Two test methods described
generated by fretting and fretting corrosion of the modular
are as follows:
interface.
1.1.1 Method I—The primary purpose of this method is to
1.5 The general concepts and methodologies described in
provide a uniform set of guidelines for long-term testing to
this practice could be applied to the study of other modular
determine the amount of damage by measurement of the
interfaces in total joint prostheses.
production of corrosion products and particulate debris from
fretting and fretting corrosion. Damage is also assessed by 1.6 The values stated in SI units are to be regarded as
characterization of the damage to the bore and cone surfaces standard. No other units of measurement are included in this
(4, 5). standard.
1.1.2 Methods II—This method provides for short-term
1.7 This standard may involve hazardous materials,
electrochemical evaluation of the fretting corrosion of the
operations, and equipment. This standard does not purport to
modular interface. It is not the intent of this method to produce
address all of the safety concerns, if any, associated with its
damage nor particulate debris but rather to provide a rapid
use. It is the responsibility of the user of this standard to
method for qualitative assessment of design changes which do
establish appropriate safety and health practices and deter-
not include material changes (1-4).
mine the applicability of regulatory limitations prior to use.
1.2 This practice does not provide for judgment or predic-
tion of in-vivo implant performance, but rather provides for a
2. Referenced Documents
uniform set of guidelines for evaluating relative differences in
3
2.1 ASTM Standards:
performance between differing implant designs, constructs, or
E4 Practices for Force Verification of Testing Machines
materials with performance defined in the context of the
E466 Practice for Conducting Force Controlled Constant
amount of fretting and fretting corrosion. Also, this practice
Amplitude Axial Fatigue Tests of Metallic Materials
should permit direct comparison of fretting corrosion data
E467 Practice for Verification of Constant Amplitude Dy-
between independent research groups, and thus provide for
namic Forces in an Axial Fatigue Testing System
building of a data base on modular implant performance.
F561 Practice for Retrieval and Analysis of Medical
1.3 This practice provides for comparative testing of manu-
Devices, and Associated Tissues and Fluids
factured hip femoral heads and stems and for coupon type
F746 Test Method for Pitting or Crevice Corrosion of
specimen testing where the male taper portion of the modular
Metallic Surgical Implant Materials
junction does not include the entire hip implant, with the taper
F897 Test Method for Measuring Fretting Corrosion of
Osteosynthesis Plates and Screws
F1440 Practice for Cyclic Fatigue Testing of Metallic
Stemmed HipArthroplasty Femoral Components Without
1
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.22 on Arthroplasty
Current edition approved Oct. 1, 2014. Published November 2014. Originally
3
approved in 1998. Last previous edition approved in 2009 as F1875 – 98(2009). For referenced ASTM standards, visit the ASTM website, www.astm.org, or
DOI: 10.1520/F1875-98R14. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
2
The bold face numbers in parentheses refers to the list of references at the end Standards volume information, refer to the standard’s Document Summary page on
of this standard. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-29
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F1875 − 98 (Reapproved 2009) F1875 − 98 (Reapproved 2014)
Standard Practice for
Fretting Corrosion Testing of Modular Implant Interfaces:
1
Hip Femoral Head-Bore and Cone Taper Interface
This standard is issued under the fixed designation F1875; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This practice describes the testing, analytical, and characterization methods for evaluating the mechanical stability of the
bore and cone interface of the head and stem junction of modular hip implants subjected to cyclic loading by measurements of
2
fretting corrosion (1-5). Two test methods described are as follows:
1.1.1 Method I—The primary purpose of this method is to provide a uniform set of guidelines for long-term testing to determine
the amount of damage by measurement of the production of corrosion products and particulate debris from fretting and fretting
corrosion. Damage is also assessed by characterization of the damage to the bore and cone surfaces (4, 5).
1.1.2 Methods II—This method provides for short-term electrochemical evaluation of the fretting corrosion of the modular
interface. It is not the intent of this method to produce damage nor particulate debris but rather to provide a rapid method for
qualitative assessment of design changes which do not include material changes (1-4).
1.2 This practice does not provide for judgment or prediction of in-vivo implant performance, but rather provides for a uniform
set of guidelines for evaluating relative differences in performance between differing implant designs, constructs, or materials with
performance defined in the context of the amount of fretting and fretting corrosion. Also, this practice should permit direct
comparison of fretting corrosion data between independent research groups, and thus provide for building of a data base on
modular implant performance.
1.3 This practice provides for comparative testing of manufactured hip femoral heads and stems and for coupon type specimen
testing where the male taper portion of the modular junction does not include the entire hip implant, with the taper portion of the
coupon identical in design, manufacturing, and materials to the taper of the final hip implant (4,5).
1.4 Method I of this practice permits simultaneous evaluation of the fatigue strength of a femoral hip stem (in accordance with
Practice F1440) and the mechanical stability and debris generated by fretting and fretting corrosion of the modular interface.
1.5 The general concepts and methodologies described in this practice could be applied to the study of other modular interfaces
in total joint prostheses.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all
of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate
safety and health practices and determine the applicability of regulatory limitations prior to use.
2. Referenced Documents
3
2.1 ASTM Standards:
E4 Practices for Force Verification of Testing Machines
E466 Practice for Conducting Force Controlled Constant Amplitude Axial Fatigue Tests of Metallic Materials
E467 Practice for Verification of Constant Amplitude Dynamic Forces in an Axial Fatigue Testing System
F561 Practice for Retrieval and Analysis of Medical Devices, and Associated Tissues and Fluids
F746 Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials
1
This practice is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee F04.15
on Material Test Methods
Current edition approved Dec. 1, 2009Oct. 1, 2014. Published December 2009November 2014. Originally approved in 1998. Last previous edition approved in 20042009
as F1875 – 98(2004).(2009). DOI: 10.1520/F1875-98R09.10.1520/F1875-98R14.
2
The bold face numbers in parentheses refers to the list of references at the end of this standard.
3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Stan
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.