ASTM F1814-22
(Guide)Standard Guide for Evaluating Modular Hip and Knee Joint Components
Standard Guide for Evaluating Modular Hip and Knee Joint Components
SIGNIFICANCE AND USE
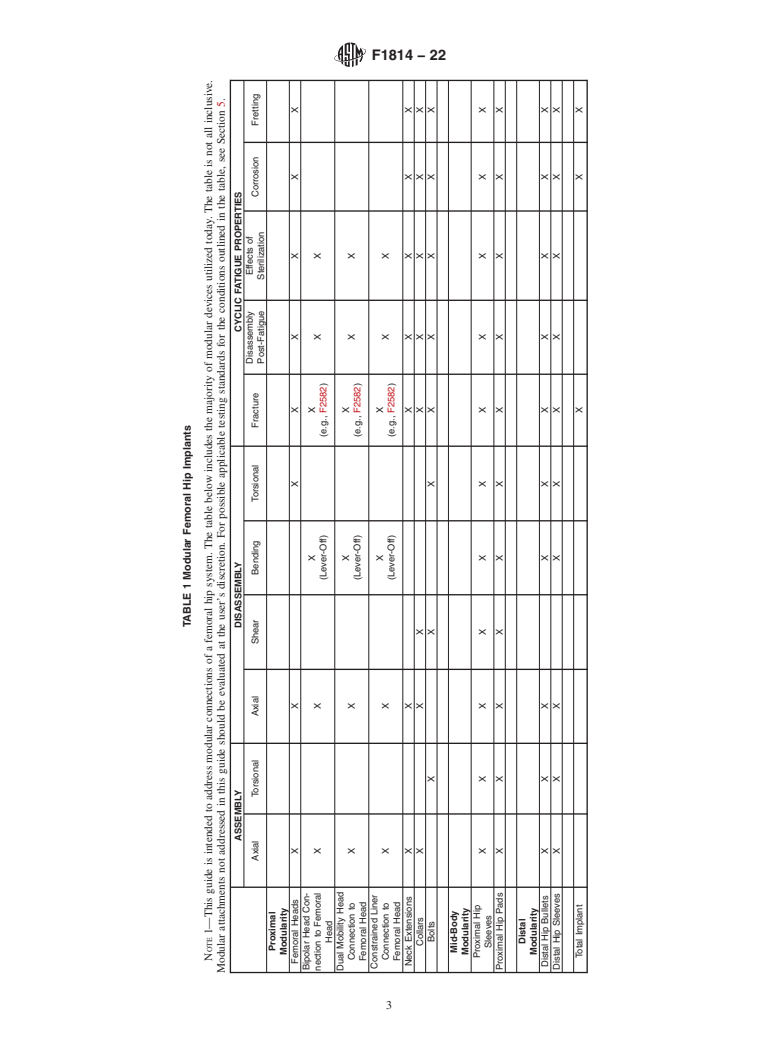
4.1 The tests suggested within this guide cover many different, but not all possible, areas of research and concern with regard to modular hip and modular knee components.
4.2 Due to the unlimited possible modular designs, this guide should be utilized as a guide for what should be considered with regard to device safety testing. There may be circumstances where alternative test methods may be useful. It is still the responsibility of the investigator to address all safety concerns that are inherent to individual modular designs.
4.3 The tests suggested herein should be utilized in such a way that the results reflect the effects of modularity, if any.
4.4 Tests that are checked in Table 1, Table 2, or Table 3 or indicated in this guide as a possible test to consider may not be applicable to every implant design.
SCOPE
1.1 This guide covers a procedure to assist the developer of a modular joint replacement implant in the choice of appropriate tests and evaluations to determine device safety.
1.2 This guide does not attempt to define all test methods associated with modular device evaluation.
1.3 The disassembly testing in this guide does not cover intentional intraoperative disassembly but is meant only to suggest testing necessary to determine inadvertent disassembly loads.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F1814 − 22
Standard Guide for
1
Evaluating Modular Hip and Knee Joint Components
This standard is issued under the fixed designation F1814; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope F1875 Practice for Fretting Corrosion Testing of Modular
Implant Interfaces: Hip Femoral Head-Bore and Cone
1.1 This guide covers a procedure to assist the developer of
Taper Interface
a modular joint replacement implant in the choice of appropri-
F2009 Test Method for Determining the Axial Disassembly
ate tests and evaluations to determine device safety.
Force of Taper Connections of Modular Prostheses
1.2 This guide does not attempt to define all test methods
F2345 Test Methods for Determination of Cyclic Fatigue
associated with modular device evaluation.
Strength of Ceramic Modular Femoral Heads
F2580 Practice for Evaluation of Modular Connection of
1.3 The disassembly testing in this guide does not cover
intentional intraoperative disassembly but is meant only to Proximally Fixed Femoral Hip Prosthesis
F2582 Test Method for Dynamic Impingement Between
suggest testing necessary to determine inadvertent disassembly
loads. Femoral and Acetabular Hip Components
F2723 Test Method for Evaluating Mobile Bearing Knee
1.4 This standard does not purport to address all of the
Tibial Baseplate/Bearing Resistance to Dynamic Disasso-
safety concerns, if any, associated with its use. It is the
ciation
responsibility of the user of this standard to establish appro-
F3090 Test Method for Fatigue Testing of Acetabular De-
priate safety, health, and environmental practices and deter-
vices for Total Hip Replacement
mine the applicability of regulatory limitations prior to use.
3
2.2 ISO Standards:
1.5 This international standard was developed in accor-
ISO 7206-4 Implants for surgery – Partial and total hip joint
dance with internationally recognized principles on standard-
prostheses – Part 4: Determination of endurance proper-
ization established in the Decision on Principles for the
ties and performance of stemmed femoral components
Development of International Standards, Guides and Recom-
ISO 7206-6 Implants for surgery – Partial and total hip joint
mendations issued by the World Trade Organization Technical
prostheses – Part 6: Endurance properties testing and
Barriers to Trade (TBT) Committee.
performance requirements of neck region of stemmed
femoral components
2. Referenced Documents
ISO 7206-10 Implants for surgery – Partial and total hip-
2
2.1 ASTM Standards:
joint prostheses – Part 10: Determination of resistance to
F648 Specification for Ultra-High-Molecular-Weight Poly-
static load of modular femoral heads
ethylene Powder and Fabricated Form for Surgical Im-
ISO 7206-13 Implants for surgery – Partial and total hip
plants
joint prostheses – Part 13: Determination of resistance to
F897 Test Method for Measuring Fretting Corrosion of
torque of head fixation of stemmed femoral components
Osteosynthesis Plates and Screws
F1800 Practice for Cyclic Fatigue Testing of Metal Tibial
3. Terminology
Tray Components of Total Knee Joint Replacements
3.1 Definitions of Terms Specific to This Standard:
F1820 Test Method for Determining the Forces for Disas-
3.1.1 modular femoral hip implant—any device that is
sembly of Modular Acetabular Devices
constructed of two or more mating parts intended for implan-
tation into the femur for the purpose of replacing the femoral
hip joint.
1
This guide is under the jurisdiction of ASTM Committee F04 on Medical and
Surgical Materials and Devices and is the direct responsibility of Subcommittee
3.1.1.1 bolts/screws—a fastener used to secure modular
F04.22 on Arthroplasty.
pieces of a femoral or tibial component.
Current edition approved July 15, 2022. Published August 2022. Originally
approved in 1997. Last previous edition approved in 2015 as F1814 – 15. DOI:
3.1.1.2 collar—medial platform located immediately distal
10.1520/F1814-22.
to the femoral neck.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F1814 − 15 F1814 − 22
Standard Guide for
1
Evaluating Modular Hip and Knee Joint Components
This standard is issued under the fixed designation F1814; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide covers a procedure to assist the developer of a modular joint replacement implant in the choice of appropriate tests
and evaluations to determine device safety.
1.2 This guide does not attempt to define all test methods associated with modular device evaluation.
1.3 This The disassembly testing in this guide does not cover intentional intraoperative disassembly but is meant only to suggest
testing necessary to determine inadvertent disassembly loads.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
F648 Specification for Ultra-High-Molecular-Weight Polyethylene Powder and Fabricated Form for Surgical Implants
F897 Test Method for Measuring Fretting Corrosion of Osteosynthesis Plates and Screws
F1800 Practice for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements
F1820 Test Method for Determining the Forces for Disassembly of Modular Acetabular Devices
F1875 Practice for Fretting Corrosion Testing of Modular Implant Interfaces: Hip Femoral Head-Bore and Cone Taper Interface
F2009 Test Method for Determining the Axial Disassembly Force of Taper Connections of Modular Prostheses
F2345 Test Methods for Determination of Cyclic Fatigue Strength of Ceramic Modular Femoral Heads
F2580 Practice for Evaluation of Modular Connection of Proximally Fixed Femoral Hip Prosthesis
F2582 Test Method for Dynamic Impingement Between Femoral and Acetabular Hip Components
F2723 Test Method for Evaluating Mobile Bearing Knee Tibial Baseplate/Bearing Resistance to Dynamic Disassociation
F3090 Test Method for Fatigue Testing of Acetabular Devices for Total Hip Replacement
3
2.2 ISO Standard:Standards:
ISO 7206-4:2010 7206-4 Implants for surgery – Partial and total hip joint prostheses – Part 4: Determination of endurance
properties and performance of stemmed femoral components
1
This guide is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee F04.22
on Arthroplasty.
Current edition approved Oct. 15, 2015July 15, 2022. Published December 2015August 2022. Originally approved in 1997. Last previous edition approved in 20092015
as F1814 – 97aF1814 – 15.(2009). DOI: 10.1520/F1814-15.10.1520/F1814-22.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F1814 − 22
ISO 7206-6:20137206-6 Implants for surgery – Partial and total hip joint prostheses – Part 6: Endurance properties testing and
performance requirements of neck region of stemmed femoral components
ISO 7206-10 Implants for surgery – Partial and total hip-joint prostheses – Part 10: Determination of resistance to static load
of modular femoral heads
ISO 7206-13 Implants for surgery – Partial and total hip joint prostheses – Part 13: Determination of resistance to torque of head
fixation of stemmed femoral components
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 modular femoral hip implant—any device that is
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.