ASTM D3523-92(2007)
(Test Method)Standard Test Method for Spontaneous Heating Values of Liquids and Solids (Differential Mackey Test)
Standard Test Method for Spontaneous Heating Values of Liquids and Solids (Differential Mackey Test)
SIGNIFICANCE AND USE
The spontaneous heating value of a substance is a measure of the ability of that substance to undergo self-heating reactions while supported by cellulosic or other fibrous material in air. It is an index of the autoignition tendency of the substance under such conditions.
SCOPE
1.1 This test method covers the non-adiabatic determination of the spontaneous heating values (SHV) of liquids and solids. It is applicable to substances that are not completely volatile at the test temperature. Spontaneous heating values obtained by this test method are qualitative indications of the degree of self-heating that may be expected to occur upon exposure of the sample to air at the test temperature.
1.2 Values obtained by this method are applicable to liquids and solids supported on cellulosic surfaces. They are not applicable to liquids on metal surfaces, on contaminated surfaces, or at pressures above atmospheric.
1.3 Spontaneous heating values determined by the present test method are regarded only as qualitative measurements of self-heating which occurs under the conditions of the test. The test method does not purport to produce a quantitative measure of the enthalpy of reaction of the sample with air at a given test temperature. Such data can be obtained by the use of an adiabatic calorimeter. The existence, under the test conditions, of a positive temperature difference between the sample and the reference is evidence of a thermochemical reaction in the sample.
1.4 The magnitude of the measured temperature difference is a semiquantitative indication of the enthalpy and rate of that reaction. Since factors such as heat loss from the sample to the bath and quenching of the reaction due to too rapid consumption of oxygen affect the amount and duration of the measured heat effect, care must be taken not to attribute too much quantitative significance to the test results. It is sufficient, for the purpose of this test, to determine whether or not the sample is capable of undergoing a self-heating reaction of sufficient magnitude and rapidity to produce a detectable thermal effect. The spontaneous heating value (SHV) can be lower than the test temperature. A negative result does not preclude spontaneous heating initiating at a temperature higher than the test temperature.
1.5 This standard should be used to measure and describe the response of materials, products, or assemblies to heat and flame under controlled conditions and should not be used to describe or appraise the fire-hazard or fire-risk of materials, products, or assemblies under actual fire conditions. However, results of this test may be used as elements of a fire-hazard assessment or a fire-risk assessment which takes into account all of the factors which are pertinent to an assessment of the fire hazard or fire risk of a particular end use.
1.6 The values stated in SI units are to be regarded as the standard. In cases where materials, products or equipment are available in inch-pound units only, SI units are omitted.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D3523–92(Reapproved 2007)
Standard Test Method for
Spontaneous Heating Values of Liquids and Solids
(Differential Mackey Test)
This standard is issued under the fixed designation D3523; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.5 This standard should be used to measure and describe
the response of materials, products, or assemblies to heat and
1.1 Thistestmethodcoversthenon-adiabaticdetermination
flame under controlled conditions and should not be used to
of the spontaneous heating values (SHV) of liquids and solids.
describe or appraise the fire-hazard or fire-risk of materials,
It is applicable to substances that are not completely volatile at
products, or assemblies under actual fire conditions. However,
the test temperature. Spontaneous heating values obtained by
results of this test may be used as elements of a fire-hazard
this test method are qualitative indications of the degree of
assessment or a fire-risk assessment which takes into account
self-heating that may be expected to occur upon exposure of
allofthefactorswhicharepertinenttoanassessmentofthefire
the sample to air at the test temperature.
hazard or fire risk of a particular end use.
1.2 Values obtained by this method are applicable to liquids
1.6 The values stated in SI units are to be regarded as the
and solids supported on cellulosic surfaces. They are not
standard. In cases where materials, products or equipment are
applicable to liquids on metal surfaces, on contaminated
available in inch-pound units only, SI units are omitted.
surfaces, or at pressures above atmospheric.
1.7 This standard does not purport to address all of the
1.3 Spontaneous heating values determined by the present
safety concerns, if any, associated with its use. It is the
test method are regarded only as qualitative measurements of
responsibility of the user of this standard to establish appro-
self-heating which occurs under the conditions of the test. The
priate safety and health practices and determine the applica-
testmethoddoesnotpurporttoproduceaquantitativemeasure
bility of regulatory limitations prior to use.
oftheenthalpyofreactionofthesamplewithairatagiventest
temperature. Such data can be obtained by the use of an
2. Referenced Documents
adiabatic calorimeter. The existence, under the test conditions,
2.1 ASTM Standards:
of a positive temperature difference between the sample and
D1193 Specification for Reagent Water
the reference is evidence of a thermochemical reaction in the
sample.
3. Terminology
1.4 The magnitude of the measured temperature difference
3.1 Definitions of Terms Specific to This Standard:
is a semiquantitative indication of the enthalpy and rate of that
3.1.1 spontaneous heating value (SHV)—the maximum
reaction. Since factors such as heat loss from the sample to the
amountbywhichthetemperatureofthesampleexceedsthatof
bath and quenching of the reaction due to too rapid consump-
the reference when exposed at a given temperature in the
tion of oxygen affect the amount and duration of the measured
standard apparatus.
heat effect, care must be taken not to attribute too much
3.2 Symbols:
quantitative significance to the test results. It is sufficient, for
thepurposeofthistest,todeterminewhetherornotthesample
is capable of undergoing a self-heating reaction of sufficient
t = temperature of sample side at any time during test,
S
magnitude and rapidity to produce a detectable thermal effect.
K,
The spontaneous heating value (SHV) can be lower than the
t = temperatureofreferencesideattime t ismeasured,
R S
test temperature. A negative result does not preclude sponta-
K,
neous heating initiating at a temperature higher than the test
T = maximum temperature of sample chamber during
S
temperature.
test, K,
This test method is under the jurisdiction of ASTM Committee D02 on
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
D02.L0.07 on Engineering Sciences of High Performance Fluids and Solids. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved May 1, 2007. Published June 2007. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1976. Last previous edition approved in 2002 as D3523–92(2002). Standards volume information, refer to the standard’s Document Summary page on
DOI: 10.1520/D3523-92R07. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D3523–92 (2007)
6. Apparatus
T = temperatureofreferencechambermeasuredatsame
R
time that T is measured, K,
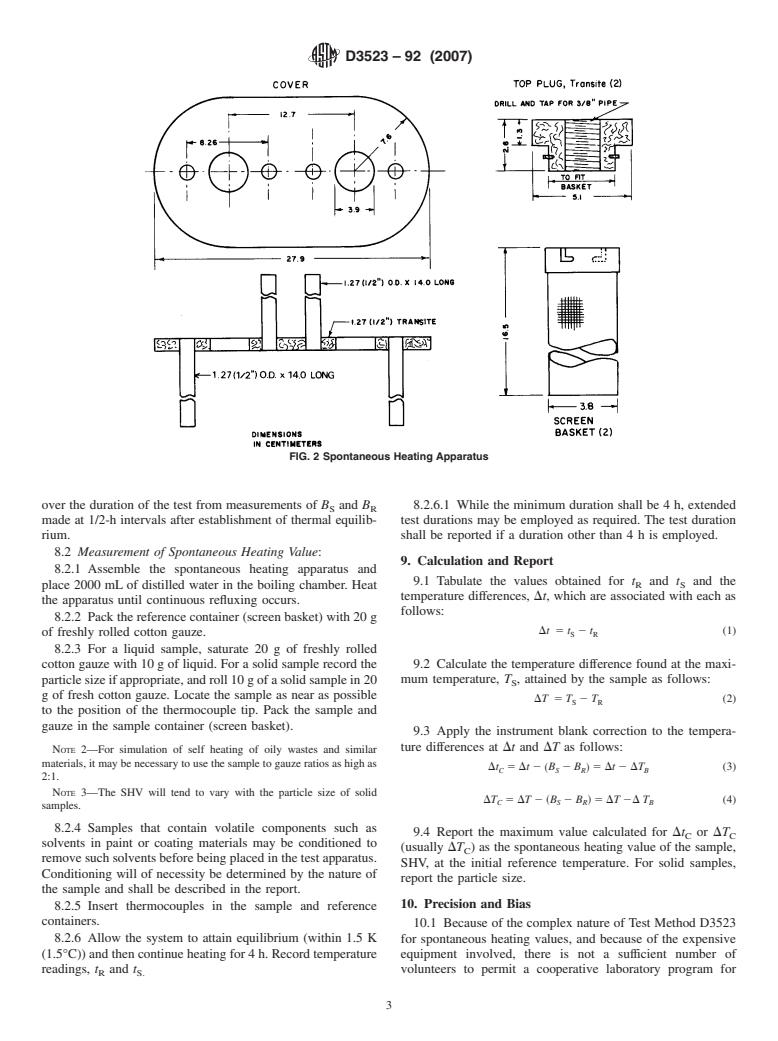
6.1 Spontaneous Heating Apparatus—SeeFig.1andFig.2.
S
Dt = t − t ,
6.2 Thermocouples, 30-gage, iron-constantan, Type J. Two
S R
DT = T − T ,
S R are required.
B = equilibrium temperature of reference side during
R
6.3 Strip Chart Temperature Recorder, two-channel or mul-
blank run, K,
tipoint, capable of 0.5 K resolution at test temperature.
B = equilibrium temperature of sample side during
S
6.4 Hot Plate, capable of uniformly heating entire bottom
blank run, K,
surface of spontaneous heating apparatus.
DT = B − B =instrumental blank,
B S R
Dt = Dt−(B −B )= Dt− DT , and
C S R B
7. Materials
DT = DT−(B − B )= DT− DT =spontaneous heating
C S R B
7.1 Cotton Gauze, surgical.
value.
7.2 Water, conforming to Specification D1193, Type III.
8. Procedure
4. Summary of Test Method
8.1 Determination of Instrumental Blank:
4.1 Thesampleissupportedonsurgicalgauzeandplacedin
8.1.1 Assemble the spontaneous heating apparatus and
a heated chamber which is open to the air at the top. The
place 2000 mL of distilled water in the boiling chamber. Heat
temperature of the sample, thus prepared, is compare
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.