ASTM F3212-16
(Test Method)Standard Test Method for Coring Testing of Huber Needles
Standard Test Method for Coring Testing of Huber Needles
SIGNIFICANCE AND USE
5.1 This test method determines whether Huber needles are designed and manufactured such that they will not produce a core during simulated implantable port access.
5.2 If a needle produces a core during actual use, leaking of the implantable port may occur. Also, the core may be flushed into the port’s reservoir and subsequently into the patient’s body.
SCOPE
1.1 This test method covers the qualitative measurement of Huber-type needles’ potential to remove septum material during implantable port access (1)2.
1.2 This test method does not address other issues that may include, but are not limited to, force measurement during the perforation/withdrawal, septum integrity, and any safety issues.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F3212 − 16
Standard Test Method for
1
Coring Testing of Huber Needles
This standard is issued under the fixed designation F3212; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.1.6 implantable port, n—a reservoir placed under the skin
(and usually attached to a catheter) that is made to receive a
1.1 This test method covers the qualitative measurement of
needle through a septum; it is often used to deliver medication.
Huber-type needles’ potential to remove septum material
2 See Fig. 2.
during implantable port access (1) .
3.1.7 lumen, n—the inside surface of the cannula.
1.2 This test method does not address other issues that may
3.1.8 septum, n—a feature of an implantable port that allows
include, but are not limited to, force measurement during the
repeated access by a port-access needle, generally composed of
perforation/withdrawal, septum integrity, and any safety issues.
an elastomeric material. See Fig. 2, Item 1.
1.3 This standard does not purport to address all of the
3.1.9 stylet, n—a device, preferably metallic, inserted into
safety concerns, if any, associated with its use. It is the
the lumen to remove a core.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
4. Summary of Test Method
bility of regulatory limitations prior to use.
4.1 A silicone elastomeric disk (surrogate septum or just
2. Referenced Documents
septum thereafter) is clamped into a specifically designed
3
septum holder. The test operator accesses the septum with a
2.1 ASTM Standards:
Huber needle in accordance with the needle manufacturer’s
D2240 Test Method for Rubber Property—Durometer Hard-
instructions for use, as if the septum was an implantable port.
ness
The lumen at the bevel is examined for the existence of a core,
3. Terminology
preferably before the needle is withdrawn. This is categorized
as a pass/fail test. Existence of a core in the needle’s cannula is
3.1 Definitions of Terms Specific to This Standard:
a failed result.
3.1.1 bevel, n—the slanted part of a needle that creates a
sharp pointed tip.
5. Significance and Use
3.1.2 cannula, n—the tubular part of a needle through which
5.1 This test method determines whether Huber needles are
fluids pass.
designed and manufactured such that they will not produce a
3.1.3 core, n—a sliver of septum material that may be
core during simulated implantable port access.
produced when a needle perforates a septum.
5.2 If a needle produces a core during actual use, leaking of
3.1.4 heel, n—the rear cutting edge of the needle bevel.
the implantable port may occur. Also, the core may be flushed
3.1.5 Huber needle, n—a needle whose tip is angles such
into the port’s reservoir and subsequently into the patient’s
that the bevel opening is parallel to the main axis of the
body.
cannula. Its special shape slices rather than perforates the
6. Apparatus
septum, reducing the chance of leakage due to coring. It is also
known as a non-coring needed. See Fig. 1.
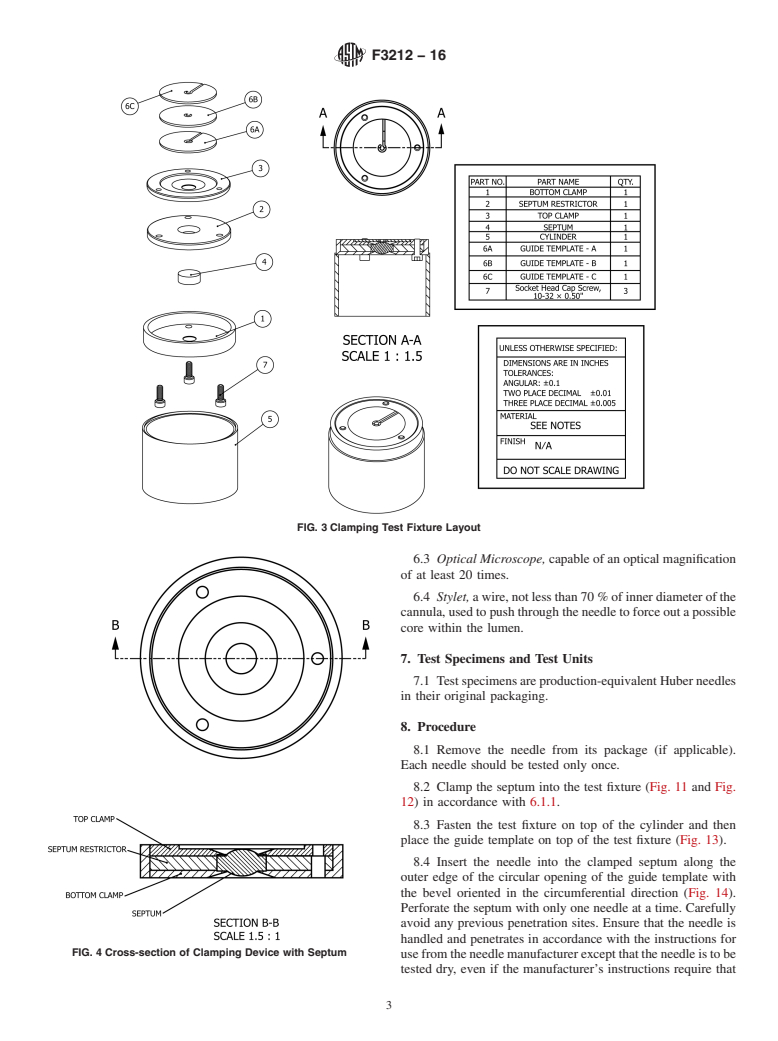
6.1 Clamping Test Fixture, a clamping device which can
hold a septum with nominal dimensions of 0.70 in. in diameter
1
and 0.25 in. thick. The clamping device is such that it restrains
This test method is under the jurisdiction of ASTM Committee F04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee radial expansion of the septum under axial compression. The
F04.33 on Medical/Surgical Instruments.
compression force is specified when the compression plates are
Current edition approved Oct. 1, 2016. Published October 2016. DOI: 10.1520/
in contact. The distance between the two compressive surfaces
F3212-16.
2
of the fixture plates after the clamping will be 0.213 in. which
The boldface numbers in parentheses refer to a list of references at the end of
this standard.
results in nominal 15 % compression. See Figs. 3-13.
3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
6.1.1 The clamping test fixture consists of 6 parts (See Figs.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3-13.). The septum (Fig. 8) is placed on the opening of the
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. septum restrictor (Fig. 6). The septum restrictor with the
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F3212 − 16
FIG. 1 Example of a Huber Needle (partial section through cannula
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.