ASTM D4875-99
(Test Method)Standard Test Methods of Polyurethane Raw Materials Determination of the Polymerized Ethylene Oxide Content of Polyether Polyols
Standard Test Methods of Polyurethane Raw Materials Determination of the Polymerized Ethylene Oxide Content of Polyether Polyols
SCOPE
1.1 Test Method A -Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR) measures polymerized ethylene oxide (EO) in ethylene oxide-propylene oxide polyethers used in flexible urethane foams and nonfoams. It is suitable for diols made from the commonly used initators and containing EO percentages greater than or equal to six. For triols initiated with glycerin and trimethylol propane, an uncorrected EO value is obtained since both initiators have protons that contribute to the EO measurement (see Note 1).
1.2 Test Method B -Carbon-13 Nuclear Magnetic Resonance Spectroscopy (13C NMR) measures the polymerized EO content of ethylene oxide-propylene oxide polyethers used in flexible urethane foams and nonfoams. It is suitable for diols and triols made from the commonly used initiators and containing EO percentages greater than or equal to six. Note 1-There are no equivalent ISO standards.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D4875–99
Standard Test Methods of
Polyurethane Raw Materials: Determination of the
Polymerized Ethylene Oxide Content of Polyether Polyols
This standard is issued under the fixed designation D4875; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope* 3.2 Definitions of Terms Specific to This Standard:
3.2.1 heteric polyol, n—a poly ether polyol in which ethyl-
1.1 Test Method A—Proton Nuclear Magnetic Resonance
ene oxide and propylene oxide units are randomly arranged.
Spectroscopy( HNMR)measurespolymerizedethyleneoxide
3.2.2 initiator, n—asubstancewithwhichethyleneoxideor
(EO) in ethylene oxide-propylene oxide polyethers used in
propylene oxide reacts to form a polyether polyol.
flexible urethane foams and nonfoams. It is suitable for diols
3.2.2.1 Discussion—one initiator unit is incorporated into
made from the commonly used initators and containing EO
each polymer or oligomer molecule.
percentagesgreaterthanorequaltosix.Fortriolsinitiatedwith
glycerin and trimethylol propane, an uncorrected EO value is
4. Summary of Test Methods
obtained since both initiators have protons that contribute to
4.1 Test Method A—The H NMR spectra of polyether
the EO measurement (see Note 1).
polyols show two groups of resonance peaks corresponding to
1.2 Test Method B—Carbon-13 Nuclear Magnetic Reso-
the methyl protons of propylene oxide (PO) and to the
nance Spectroscopy ( C NMR) measures the polymerized EO
methylene and methine protons of EO and PO. The EO peak
content of ethylene oxide-propylene oxide polyethers used in
areaisobtainedbysubtractingtheareaofthePOmethylpeaks
flexible urethane foams and nonfoams. It is suitable for diols
from the area of the methylene and methine peaks. Initiators
and triols made from the commonly used initiators and
other than glycols of EO and PO give systematic errors (see
containing EO percentages greater than or equal to six.
Note 2).
NOTE 1—There are no equivalent ISO standards.
NOTE 2—The initiator error can be estimated by calculating the
1.3 This standard does not purport to address all of the
theoretical contribution of initiator protons to the EO and PO peak areas.
safety concerns, if any, associated with its use. It is the
4.2 Test Method B—The C NMR spectra of polyethers
responsibility of the user of this standard to establish appro-
contain multiple resonances arising from initiator, alkoxide,
priate safety and health practices and determine the applica-
alkoxide sequencing, and end-group distribution. EO content
bility of regulatory limitations prior to use.
may be determined relative to PO or relative to PO and triol
initiator.Intheformer,theareaoftheEOpeaksisratioedtothe
2. Referenced Documents
total area of alkoxide methylene and methine carbons. In the
2.1 ASTM Standards:
latter, the area of the EO peaks is ratioed to the total area of
D883 Terminology Relating to Plastics
alkoxide methylene and methine carbons and two initiator
E180 Practice for Developing the Precision Data ofASTM
carbons. This test method describes the determination of EO
Methods for Analysis and Testing of Industrial Chemicals
relative to PO only.
E691 Practice for Conducting an Interlaboratory Study to
Determine the Precision of a Test Method
5. Significance and Use
5.1 Measurements of EO content correlate with polyol
3. Terminology
reactivity (as related to primary hydroxyl content), linearity of
3.1 Definitions—Terminology in these test methods follows
foam rise, and the hydrophilicity of the polyol and final
the standard terminology defined in Terminology D883.
product.
5.2 Statistical data suggest that the C NMR test method is
These test methods are under the jurisdiction of ASTM Committee D-20 on
thepreferredmethodformeasuringlowlevels(lessthan10%)
Plastics and are the direct responsibility of Subcommittee D20.22 on Cellular
of polymerized EO in polyols.
Plastics.
1 13
5.3 The Hand CNMRtestmethodsgivedifferentresults
Current edition approved Nov. 10, 1999. Published February 2000. Originally
published as D4875–88. Last previous edition D4875–94.
which are highly correlated. The equation of the linear regres-
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
sion is:
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on %EO 51.031 ~%EO ! 10.883 (1)
proton carbon213
the ASTM website.
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D4875–99
The standard deviation of the regression is 0.49 and the saturation. See Figs. 1 and 2 for examples of polyol spectra
multiple R-square is 0.9990. with high and low EO concentrations, respectively.
11.2 For FTNMR, acquire the desired number of transients
TEST METHOD A—HYDROGEN-1 NMR
and transform the free induction decay signal to the frequency
domain spectrum. Integrate the spectrum.
6. Equipment
6.1 NMR Continuous Wave (CW) or Fourier Transform
12. Calculation
(FT) Spectrometer,withan Hresonancefrequencyof60MHz
12.1 Determine the areas of the PO methyl protons (areaA)
or higher.
and the EO and PO methylene and methine protons (area B)
6.2 NMR Sample Tubes, having an outside diameter of at
from the integration curves. Calculate the percent EO from the
least 5 mm.
following equation:
33.1 3 Z
7. Reagents and Materials
EO 5 3100 (2)
33.1 3 Z 158.1
7.1 All reagents should be ACS certified or spectroscopic
grade unless otherwise specified.
where:
7.2 Trifluoroacetic Acid.
Z =(B/A)−1
7.3 Chloroform-d , NMR-grade, containing tetramethylsi-
13. Report
lane as an internal standard.
13.1 Report results to the nearest tenth percent EO.
8. Standard
8.1 Thistestmethoddoesnotrequirestandards.Toevaluate 14. Precision and Bias
thetestmethod,standardsmaybepreparedfromcommercially
14.1 Table1isbasedonaroundrobinconductedin1981in
available poly(propylene oxide) and poly(ethylene oxide).
accordance with Practice E691, involving six polyol samples
with EO content ranging from 6 to 45 weight % (see Table 2)
9. Preparation of Sample
testedbyeightlaboratories.Foreachpolyol,allofthesampless
9.1 Mixafewdropsofpolyolwithdeuteratedchloroformto
werepreparedatonesource,buttheindividualspecimenswere
prepare 1 mLof an approximately 10% polyol solution.Add
prepared at the laboratories that tested them. Each test result
adropoftrifluoroaceticacid,mixwell,andtransfertoanNMR
wasobtainedfromoneindividualNMRrun.Eachlabobtained
tube.
two test results for each material on two separate days.
14.2 InTable 1, for the polyols indicated and for test results
10. Instrument Preparation
that are derived from testing two specimens of each polyol on
10.1 The instrument settings given here are for a Varian
each of two separate days:
EM-390 CW spectrometer and a Varian XL-100 FT spectrom-
14.2.1 S is the within-laboratory standard deviation of the
r
eter. Instrument preparation may vary with the spectrometer.
average: I =2.83 S (see 14.2.3 for application of I ).
r r r
For a description of a particular spectrometer and suitable
14.2.2 S is the between-laboratory standard deviation of
R
parameters, refer to the manufacturer’s operating manual.
the average; I =2.83 S (see 14.2.4 for application of I ).
R R R
10.2 Typical EM-390 console settings are as follows:
14.2.3 Repeatability—In comparing two test results for the
Lock optional, TMS
same polyol, obtained by the same operator using the same
Offset 0
Sweep width 5 ppm
Sweep time 2 min
Integration time 2 min
Rf Filter open
RF power 0.05 mG
10.3 Typical XL-100 console settings are as follows:
Lock chloroform-d
Pulse width 90°
Pulse delay 0
Spectra width 10 ppm
Aquisition time 4 sec
Data points 8K
Number of transients 128
11. NMR Analysis
11.1 PlacetheNMRtubecontainingthepolyolsolutioninto
thespectrometerprobeandoptimizethefieldhomogeneity.For
CW NMR, scan the spectrum from 5 to 0 ppm. Integrate the
spectrum five times at a power level below that which causes
Highfield, FT spectrophotometers require less concentrated solutions. A 1%
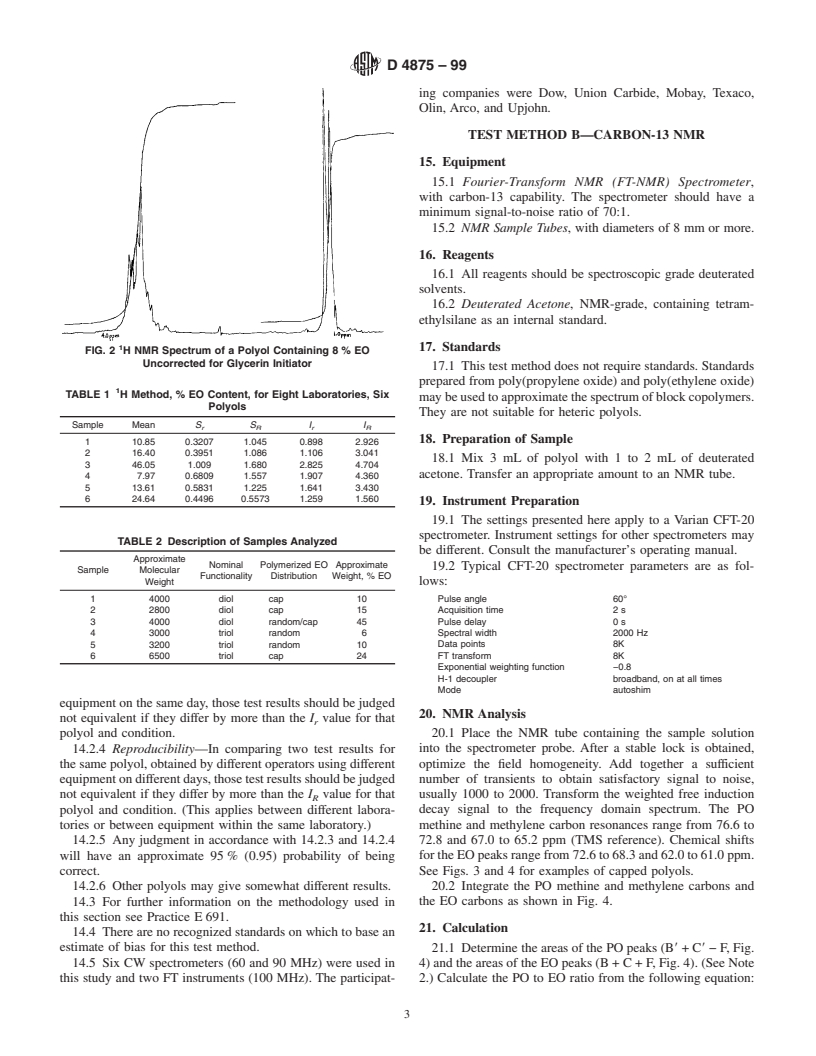
solution is more appropriate for such spectrophotometers. FIG. 1 H NMR Spectrum of a Polyol Containing 45% EO
D4875–99
ing companies were Dow, Union Carbide, Mobay, Texaco,
Olin, Arco, and Upjohn.
TEST METHOD B—CARBON-13 NMR
15. Equipment
15.1 Fourier-Transform NMR (FT-NMR) Spectrometer,
with carbon-13 capability. The spectrometer should have a
minimum signal-to-noise ratio of 70:1.
15.2 NMR Sample Tubes, with diameters of 8 mm or more.
16. Reagents
16.1 All reagents should be spectroscopic grade deuterated
solvents.
16.2 Deuterated Acetone, NMR-grade, containing tetram-
ethylsilane as an internal standard.
17. Standards
FIG. 2 H NMR Spectrum of a Polyol Containing 8% EO
Uncorrected for Glycerin Initiator
17.1 This test method does not require standards. Standards
prepared from poly(propylene oxide) and poly(ethylene oxide)
TABLE 1 H Method, % EO Content, for Eight Laboratories, Six
maybeusedtoapproximatethespectrumofblockcopolymers.
Polyols
They are not suitabl
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.