ASTM G148-97(2003)
(Practice)Standard Practice for Evaluation of Hydrogen Uptake, Permeation, and Transport in Metals by an Electrochemical Technique
Standard Practice for Evaluation of Hydrogen Uptake, Permeation, and Transport in Metals by an Electrochemical Technique
SIGNIFICANCE AND USE
The procedures described, herein, can be used to evaluate the severity of hydrogen charging of a material produced by exposure to corrosive environments or by cathodic polarization. It can also be used to determine fundamental properties of materials in terms of hydrogen diffusion (for example, diffusivity of hydrogen) and the effects of metallurgical, processing, and environmental variables on diffusion of hydrogen in metals.
The data obtained from hydrogen permeation tests can be combined with other tests related to hydrogen embrittlement or hydrogen induced cracking to ascertain critical levels of hydrogen flux or hydrogen content in the material for cracking to occur.
SCOPE
1.1 This practice gives a procedure for the evaluation of hydrogen uptake, permeation, and transport in metals using an electrochemical technique which was developed by Devanathan and Stachurski. While this practice is primarily intended for laboratory use, such measurements have been conducted in field or plant applications. Therefore, with proper adaptations, this practice can also be applied to such situations.
1.2 This practice describes calculation of an effective diffusivity of hydrogen atoms in a metal and for distinguishing reversible and irreversible trapping.
1.3 This practice specifies the method for evaluating hydrogen uptake in metals based on the steady-state hydrogen flux.
1.4 This practice gives guidance on preparation of specimens, control and monitoring of the environmental variables, test procedures, and possible analyses of results.
1.5 This practice can be applied in principle to all metals and alloys which have a high solubility for hydrogen, and for which the hydrogen permeation is measurable. This method can be used to rank the relative aggressivity of different environments in terms of the hydrogen uptake of the exposed metal.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:G148–97 (Reapproved 2003)
Standard Practice for
Evaluation of Hydrogen Uptake, Permeation, and Transport
in Metals by an Electrochemical Technique
This standard is issued under the fixed designation G148; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope G96 Guide for Online Monitoring of Corrosion in Plant
Equipment (Electrical and Electrochemical Methods)
1.1 This practice gives a procedure for the evaluation of
hydrogen uptake, permeation, and transport in metals using an
3. Terminology
electrochemical technique which was developed by Devana-
2 3.1 Definitions:
than and Stachurski. While this practice is primarily intended
3.1.1 charging,, n—method of introducing atomic hydrogen
for laboratory use, such measurements have been conducted in
into the metal by galvanostatic charging (constant charging
field or plant applications. Therefore, with proper adaptations,
current), potentiostatic charging (constant electrode potential),
this practice can also be applied to such situations.
free corrosion, or gaseous exposure.
1.2 This practice describes calculation of an effective diffu-
3.1.2 charging cell,, n—compartment in which hydrogen
sivity of hydrogen atoms in a metal and for distinguishing
atoms are generated on the specimen surface. This includes
reversible and irreversible trapping.
both aqueous and gaseous charging.
1.3 This practice specifies the method for evaluating hydro-
3.1.3 decay current,, n—decay of the hydrogen atom oxi-
gen uptake in metals based on the steady-state hydrogen flux.
dation current due to a decrease in charging current.
1.4 This practice gives guidance on preparation of speci-
3.1.4 Fick’s second law,, n—second order differential equa-
mens, control and monitoring of the environmental variables,
tion describing the concentration of diffusing specie as a
test procedures, and possible analyses of results.
function of position and time. The equation is of the form
1.5 This practice can be applied in principle to all metals
]C~x,t!/]t 5]/]xD ]/]x[C~x,t!# for lattice diffusion in one
and alloys which have a high solubility for hydrogen, and for
dimension where diffusivity is independent of concentration.
which the hydrogen permeation is measurable. This method
See 3.2 for symbols.
can be used to rank the relative aggressivity of different
3.1.5 hydrogen flux,, n—the amount of hydrogen passing
environments in terms of the hydrogen uptake of the exposed
through the metal specimen per unit area as a function of time.
metal.
The units are typically concentration per unit area per unit
1.6 This standard does not purport to address all of the
time.
safety concerns, if any, associated with its use. It is the
3.1.6 hydrogen uptake,, n—the concentration of hydrogen
responsibility of the user of this standard to establish appro-
3 3
absorbed into the metal (for example, g/cm or mol/cm ).
priate safety and health practices and determine the applica-
3.1.7 irreversible trap,, n—microstructural site at which a
bility of regulatory limitations prior to use.
hydrogen atom has a infinite or extremely long residence time
2. Referenced Documents compared to the time-scale for permeation testing at the
3 relevant temperature, as a result of a binding energy which is
2.1 ASTM Standards:
large relative to the migration energy for diffusion.
3.1.8 reversible trap,, n—microstructural site at which a
hydrogen atom has a residence time which is greater than that
This practice is under the jurisdiction of ASTM Committee G01 on Corrosion
of Metals and is the direct responsibility of Subcommittee G01.11 on Electrochemi- for the lattice site but is small in relation to the time to attain
cal Measuremnents in Corrosion Testing.
steady-state permeation, as a result of low binding energy.
Current edition approvedApr. 10, 1997. Published January 1998. DOI: 10.1520/
3.1.9 mobile hydrogen atoms,, n—hydrogen atoms that are
G0148-97R03.
associated with sites within the lattice.
Devanathan, M.A.V. and Stachurski, Z., Proceedings of Royal Society, A270,
90–102, 1962.
3.1.10 oxidation cell,, n—compartment in which hydrogen
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
atoms exiting from the metal specimen are oxidized.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3.1.11 permeation current,, n—current measured in oxida-
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. tion cell associated with oxidation of hydrogen atoms.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
G148–97 (2003)
3.1.12 permeation transient,, n—the increase of the perme- side of the membrane which eliminates the need for a charging
ation current with time from commencement of charging to the cell. See 7.1 for guidance on various specimen configurations.
attainment of steady state, or modification of charging condi-
4.2 In gaseous environments, the hydrogen atoms are gen-
tions (that is, rise transient). The decrease of the permeation
eratedbyadsorptionanddissociationofthegaseousspecies.In
current with time resulting from a decrease in charging current
aqueous environments, hydrogen atoms are produced by elec-
(that is, decay transient).
trochemical reactions. In both cases, some of the hydrogen
3.1.13 recombination poison, n—chemical specie present
atoms diffuse through the membrane and are then oxidized on
within the test environment in the charging cell which en-
exiting from the other side of the metal in the oxidation cell.
hances hydrogen absorption by retarding the recombination of
4.3 The conditions (for example, environment and the
hydrogen atoms adsorbed onto the metal surface into hydrogen
electrode potential) on the oxidation side of the membrane are
gas.
controlled so that the metal surface is either passive or immune
3.2 Symbols:
to corrosion. The background current established under these
3.2.1 For the purposes of this practice the following sym-
conditions prior to hydrogen transport should be relatively
bols apply:
constant and small compared to that of the hydrogen atom
oxidation current.
A = exposed area of specimen in the oxidation cell
4.4 The electrode potential of the specimen in the oxidation
(cm )
cell is controlled at a value sufficiently positive to ensure that
C(x,t) = lattice concentration of hydrogen as a function of
the kinetics of oxidation of hydrogen atoms are limited by the
position and time (mol/cm )
flux of hydrogen atoms, that is, the oxidation current density is
C = sub-surface concentration of atomic hydrogen at
diffusion limited.
the charging side of the specimen (mol/cm )
4.5 The total oxidation current is monitored as a function of
D = effective diffusivity of atomic hydrogen, taking
eff
time. The total oxidation current comprises the background
into account the presence of reversible and irre-
current and the current resulting from oxidation of hydrogen
versible trapping (cm /s)
atoms. The latter is the permeation current.
D = lattice diffusion coefficient of atomic hydrogen
l
(cm /s)
4.6 The thickness of the specimen is selected usually to
F = faraday’s constant (9.6485 x 10 coulombs/mol)
ensure that the measured flux reflects volume (bulk) controlled
I(t) = time dependent atomic hydrogen permeation cur-
hydrogen atom transport. Thin specimens may be used for
rent (µA)
evaluation of the effect of surface processes on hydrogen entry
I = steady-state atomic hydrogen permeation current
ss
or exit (absorption kinetics or transport in oxide films).
(µA)
4.7 In reasonably pure, defect-free metals (for example,
J(t) = time-dependent atomic hydrogen permeation flux
single crystals) with a sufficiently low density of microstruc-
asmeasuredontheoxidationsideofthespecimen
tural trap sites, atomic hydrogen transport through the material
(mol/s/cm )
is controlled by lattice diffusion.
J = atomic hydrogen permeation flux at steady-state
ss
(mol/s/cm )
4.8 Alloying and microstructural features such as disloca-
J(t)/J = normalized flux of atomic hydrogen
tions, grain boundaries, inclusions, and precipitate particles
ss
L = specimen thickness (cm)
may act as trap sites for hydrogen thus delaying hydrogen
t = time elapsed from commencement of hydrogen
transport.Thesetrapsmaybereversibleorirreversibledepend-
charging (s)
ing on the binding energy associated with the particular trap
t = elapsed time measured extrapolating the linear
b
sites compared to the energy associated with migration for
portion of the rising permeation current transient
hydrogen in the metal lattice.
to J(t)=O (s)
4.9 The rate of hydrogen atom transport through the metal
t = time to achieve a value of J(t)/J = 0.63 (s)
lag ss
during the first permeation may be affected by both irreversible
x = distance into specimen from the charging surface
and reversible trapping as well as by the reduction of any
measured in the thickness direction (cm ).
oxidespresentonthechargingsurface.Atsteadystateallofthe
t = normalized time (D t/L )
t = Normalized time to achieve a value of j(t)/J = irreversible traps are occupied. If the mobile hydrogen atoms
lag ss
are then removed and a subsequent permeation test conducted
0.63 (s)
on the specimen the difference between the first and second
4. Summary of Practice permeation transients can be used to evaluate the influence of
irreversibletrappingontransport,assuminganegligibleroleof
4.1 The technique involves locating the metal membrane
oxide reduction.
(that is, specimen) of interest between the hydrogen charging
4.10 For some environments, the conditions on the charging
andoxidationcells.Inthelaboratory,thechargingcellcontains
side of the specimen may be suitably altered to induce a decay
the environment of interest. Hydrogen atoms are generated on
of the oxidation current after attainment of steady state. The
the membrane surface exposed to this environment. In field or
plant measurements, the wall of the pipe or vessel can be used rate of decay will be determined by diffusion and reversible
trappingonlyand,hence,canalsobeusedtoevaluatetheeffect
asthemembranethroughwhichmeasurementofhydrogenflux
are made. The actual process environment is on the charging of irreversible trapping on transport during the first transient.
G148–97 (2003)
4.11 Comparison of repeated permeation transients with against the specimen and the flux exiting this additional
those obtained for the pure metal can be used in principle to material is measured may be used provided that it is demon-
evaluate the effect of reversible trapping on atomic hydrogen
strated that the introduction of this additional interface has no
transport.
effect on the calculated diffusivity. The clamping of this
4.12 This practice is suitable for systems in which hydrogen
additionalmaterialmayprovideinaccuratepermeationcurrents
atoms are generated uniformly over the charging surface of the
in some systems due to the barrier effect at the interface (that
membrane. It is not usually applicable for evaluation of
is, oxides, air gaps and so forth will act as a diffusion barrier).
corroding systems in which pitting attack occurs unless the
6.2 Non-metallic materials which are inert to the test envi-
charging cell environment is designed to simulate the localized
ronment should be used for cell construction.
pit environment and the entire metal charging surface is active.
6.2.1 At temperatures above 50°C, leaching from the cell
4.13 This practice can be used for stressed and unstressed
material (for example, silica dissolution from glass in some
specimens but testing of stressed specimens requires consider-
environments) can modify the solution chemistry and may
ation of loading procedures.
influence hydrogen permeation.
5. Significance and Use
6.2.2 Polytetrafluoroethylene (PTFE) is an example of a
material suitable for elevated temperatures up to about 90°C.
5.1 The procedures described, herein, can be used to evalu-
atetheseverityofhydrogenchargingofamaterialproducedby
6.2.3 Where metallic chambers are necessary (for contain-
exposure to corrosive environments or by cathodic polariza-
ment of high pressure environments), the materials chosen
tion. It can also be used to determine fundamental properties of
shall have a very low passive current to ensure minimal effect
materials in terms of hydrogen diffusion (for example, diffu-
on the solution composition and shall be electrically isolated
sivityofhydrogen)andtheeffectsofmetallurgical,processing,
from the membrane.
and environmental variables on diffusion of hydrogen in
6.3 The O-ring seal material should be selected to minimize
metals.
possible degradation products from the seals and contamina-
5.2 The data obtained from hydrogen permeation tests can
tion of the solution. This problem is particularly of concern
becombinedwithothertestsrelatedtohydrogenembrittlement
with highly aggressive environments and at high test tempera-
or hydrogen induced cracking to ascertain critical levels of
tures.
hydrogen flux or hydrogen content in the material for cracking
6.4 Doublejunctionreferenceelectrodesmaybeusedwhere
to occur.
necessary to avoid contamination of test solutions.At elevated
6. Apparatus temperatures,theuseofasolutionconductivitybridgearrange-
ment with suitable inert materials is recommended.
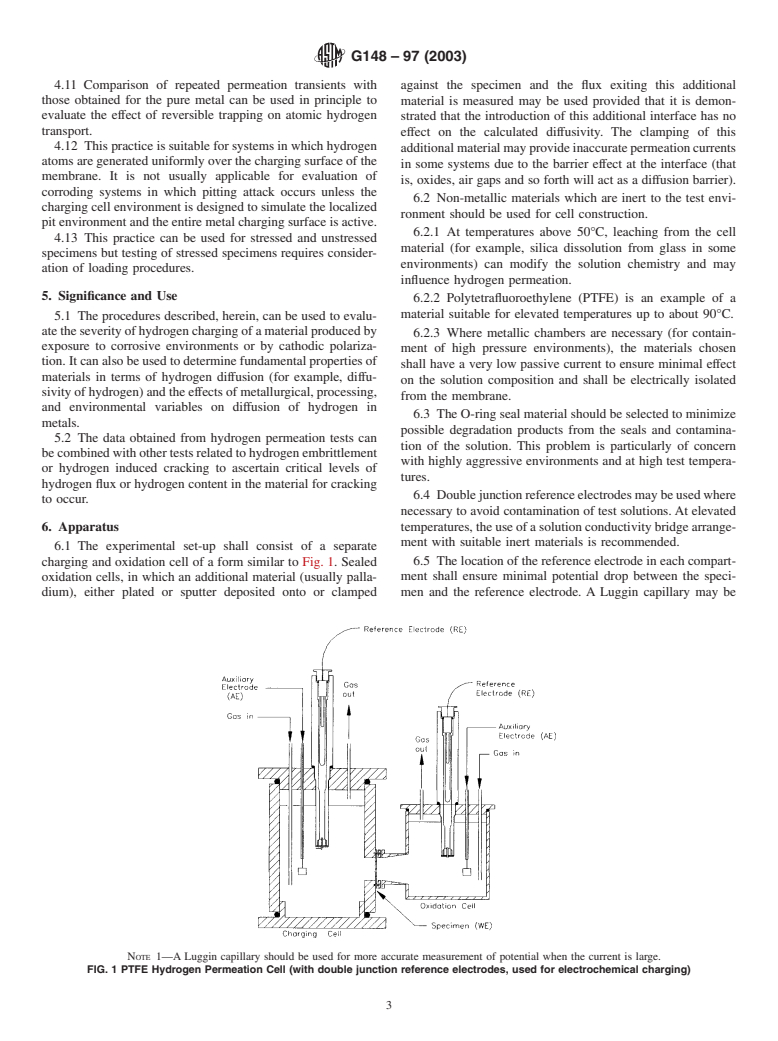
6.1 The experimental set-up shall consist of a separate
charging and oxidation cell of a form similar to Fig. 1. Sealed 6.5 The location of the reference electrode in each compart-
ment shall ensure minimal potential drop between the speci-
oxidation cells, in which an additional material (usually palla-
dium), either plated or sputter deposited onto or clamped men and the reference electrode. A Luggin capillary may be
NOTE 1—A Luggin capillary should be used for more accurate measurement of potential when the current is large.
FIG. 1 PTFE Hydrogen Permeation Cell (with double junction reference electrodes, used for electrochemical charging)
G148–97 (2003)
useful in cases where the solution resistivity is high, small cell 7.2.6 The thickness of the specimen in the region of interest
volumes are used and long tests are conducted. See Guide G96 shall be as uniform as possible with a maximum variation not
for further guidance. greater than 65%.
6.6 Recording of oxidation (and, as appropriate, charging)
7.2.7 The oxidation side of the specimen shall be mechani-
curren
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.