ASTM G148-97(2018)
(Practice)Standard Practice for Evaluation of Hydrogen Uptake, Permeation, and Transport in Metals by an Electrochemical Technique
Standard Practice for Evaluation of Hydrogen Uptake, Permeation, and Transport in Metals by an Electrochemical Technique
SIGNIFICANCE AND USE
5.1 The procedures described, herein, can be used to evaluate the severity of hydrogen charging of a material produced by exposure to corrosive environments or by cathodic polarization. It can also be used to determine fundamental properties of materials in terms of hydrogen diffusion (for example, diffusivity of hydrogen) and the effects of metallurgical, processing, and environmental variables on diffusion of hydrogen in metals.
5.2 The data obtained from hydrogen permeation tests can be combined with other tests related to hydrogen embrittlement or hydrogen induced cracking to ascertain critical levels of hydrogen flux or hydrogen content in the material for cracking to occur.
SCOPE
1.1 This practice gives a procedure for the evaluation of hydrogen uptake, permeation, and transport in metals using an electrochemical technique which was developed by Devanathan and Stachurski.2 While this practice is primarily intended for laboratory use, such measurements have been conducted in field or plant applications. Therefore, with proper adaptations, this practice can also be applied to such situations.
1.2 This practice describes calculation of an effective diffusivity of hydrogen atoms in a metal and for distinguishing reversible and irreversible trapping.
1.3 This practice specifies the method for evaluating hydrogen uptake in metals based on the steady-state hydrogen flux.
1.4 This practice gives guidance on preparation of specimens, control and monitoring of the environmental variables, test procedures, and possible analyses of results.
1.5 This practice can be applied in principle to all metals and alloys which have a high solubility for hydrogen, and for which the hydrogen permeation is measurable. This method can be used to rank the relative aggressivity of different environments in terms of the hydrogen uptake of the exposed metal.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: G148 − 97 (Reapproved 2018)
Standard Practice for
Evaluation of Hydrogen Uptake, Permeation, and Transport
in Metals by an Electrochemical Technique
This standard is issued under the fixed designation G148; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This practice gives a procedure for the evaluation of 2.1 ASTM Standards:
hydrogen uptake, permeation, and transport in metals using an G96Guide for Online Monitoring of Corrosion in Plant
electrochemical technique which was developed by Devana- Equipment (Electrical and Electrochemical Methods)
than and Stachurski. While this practice is primarily intended
for laboratory use, such measurements have been conducted in 3. Terminology
field or plant applications. Therefore, with proper adaptations,
3.1 Definitions:
this practice can also be applied to such situations.
3.1.1 charging, n—method of introducing atomic hydrogen
into the metal by galvanostatic charging (constant charging
1.2 This practice describes calculation of an effective diffu-
sivity of hydrogen atoms in a metal and for distinguishing current), potentiostatic charging (constant electrode potential),
free corrosion, or gaseous exposure.
reversible and irreversible trapping.
3.1.2 charging cell, n—compartment in which hydrogen
1.3 This practice specifies the method for evaluating hydro-
atoms are generated on the specimen surface. This includes
gen uptake in metals based on the steady-state hydrogen flux.
both aqueous and gaseous charging.
1.4 This practice gives guidance on preparation of
3.1.3 decay current, n—decay of the hydrogen atom oxida-
specimens, control and monitoring of the environmental
tion current due to a decrease in charging current.
variables, test procedures, and possible analyses of results.
3.1.4 Fick’s second law, n—second order differential equa-
1.5 This practice can be applied in principle to all metals
tion describing the concentration of diffusing specie as a
and alloys which have a high solubility for hydrogen, and for
function of position and time. The equation is of the form
which the hydrogen permeation is measurable. This method
]C x,t /]t5]/]xD ]/]x C x,t for lattice diffusion in one di-
~ ! @ ~ !#
can be used to rank the relative aggressivity of different
mensionwherediffusivityisindependentofconcentration.See
environments in terms of the hydrogen uptake of the exposed
3.2 for symbols.
metal.
3.1.5 hydrogen flux, n—the amount of hydrogen passing
1.6 This standard does not purport to address all of the
through the metal specimen per unit area as a function of time.
safety concerns, if any, associated with its use. It is the
The units are typically concentration per unit area per unit
responsibility of the user of this standard to establish appro-
time.
priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use. 3.1.6 hydrogen uptake, n—the concentration of hydrogen
3 3
absorbed into the metal (for example, g/cm or mol/cm ).
1.7 This international standard was developed in accor-
dance with internationally recognized principles on standard-
3.1.7 irreversible trap, n—microstructural site at which a
ization established in the Decision on Principles for the
hydrogen atom has a infinite or extremely long residence time
Development of International Standards, Guides and Recom-
compared to the time-scale for permeation testing at the
mendations issued by the World Trade Organization Technical
relevant temperature, as a result of a binding energy which is
Barriers to Trade (TBT) Committee.
large relative to the migration energy for diffusion.
3.1.8 mobile hydrogen atoms, n—hydrogen atoms that are
associated with sites within the lattice.
This practice is under the jurisdiction ofASTM Committee G01 on Corrosion
ofMetalsandisthedirectresponsibilityofSubcommitteeG01.11onElectrochemi-
cal Measurements in Corrosion Testing.
Current edition approved May 1, 2018. Published June 2018. Last previous For referenced ASTM standards, visit the ASTM website, www.astm.org, or
edition approved in 2011 as G148–97 (2011). DOI:10.1520/G0148-97R18. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Devanathan, M.A.V., and Stachurski, Z., Proceedings of Royal Society, A270, Standards volume information, refer to the standard’s Document Summary page on
90–102, 1962. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G148 − 97 (2018)
3.1.9 oxidation cell, n—compartment in which hydrogen 4. Summary of Practice
atoms exiting from the metal specimen are oxidized.
4.1 The technique involves locating the metal membrane
(that is, specimen) of interest between the hydrogen charging
3.1.10 permeation current, n—current measured in oxida-
andoxidationcells.Inthelaboratory,thechargingcellcontains
tion cell associated with oxidation of hydrogen atoms.
the environment of interest. Hydrogen atoms are generated on
3.1.11 permeation transient, n—the increase of the perme-
the membrane surface exposed to this environment. In field or
ationcurrentwithtimefromcommencementofchargingtothe
plant measurements, the wall of the pipe or vessel can be used
attainment of steady state, or modification of charging condi-
asthemembranethroughwhichmeasurementofhydrogenflux
tions (that is, rise transient). The decrease of the permeation
are made. The actual process environment is on the charging
current with time resulting from a decrease in charging current
sideofthemembranewhicheliminatestheneedforacharging
(that is, decay transient).
cell. See 7.1 for guidance on various specimen configurations.
3.1.12 recombination poison, n—chemical specie present
4.2 In gaseous environments, the hydrogen atoms are gen-
within the test environment in the charging cell which en-
eratedbyadsorptionanddissociationofthegaseousspecies.In
hances hydrogen absorption by retarding the recombination of
aqueous environments, hydrogen atoms are produced by elec-
hydrogenatomsadsorbedontothemetalsurfaceintohydrogen
trochemical reactions. In both cases, some of the hydrogen
gas.
atoms diffuse through the membrane and are then oxidized on
exiting from the other side of the metal in the oxidation cell.
3.1.13 reversible trap, n—microstructural site at which a
hydrogen atom has a residence time which is greater than that
4.3 The conditions (for example, environment and the
for the lattice site but is small in relation to the time to attain
electrode potential) on the oxidation side of the membrane are
steady-state permeation, as a result of low binding energy.
controlledsothatthemetalsurfaceiseitherpassiveorimmune
to corrosion. The background current established under these
3.2 Symbols:
conditions prior to hydrogen transport should be relatively
3.2.1 For the purposes of this practice the following sym-
constant and small compared to that of the hydrogen atom
bols apply:
oxidation current.
A = exposed area of specimen in the oxidation cell
4.4 The electrode potential of the specimen in the oxidation
(cm )
cell is controlled at a value sufficiently positive to ensure that
C(x,t) = lattice concentration of hydrogen as a function of
the kinetics of oxidation of hydrogen atoms are limited by the
position and time (mol/cm )
fluxofhydrogenatoms,thatis,theoxidationcurrentdensityis
C = sub-surface concentration of atomic hydrogen at
diffusion limited.
the charging side of the specimen (mol/cm )
4.5 Thetotaloxidationcurrentismonitoredasafunctionof
D = effective diffusivity of atomic hydrogen, taking
eff
time. The total oxidation current comprises the background
into account the presence of reversible and irre-
current and the current resulting from oxidation of hydrogen
versible trapping (cm /s)
atoms. The latter is the permeation current.
D = lattice diffusion coefficient of atomic hydrogen
l
(cm /s)
4.6 The thickness of the specimen is selected usually to
F = faraday’s constant (9.6485 × 10 coulombs/mol)
ensure that the measured flux reflects volume (bulk) controlled
I(t) = time dependent atomic hydrogen permeation cur-
hydrogen atom transport. Thin specimens may be used for
rent (µA)
evaluation of the effect of surface processes on hydrogen entry
I = steady-state atomic hydrogen permeation current
ss
or exit (absorption kinetics or transport in oxide films).
(µA)
J(t) = time-dependent atomic hydrogen permeation flux 4.7 In reasonably pure, defect-free metals (for example,
as measured on the oxidation side of the specimen single crystals) with a sufficiently low density of microstruc-
(mol/s/cm ) tural trap sites, atomic hydrogen transport through the material
J = atomic hydrogen permeation flux at steady-state
is controlled by lattice diffusion.
ss
(mol/s/cm )
4.8 Alloying and microstructural features such as
J(t)/J = normalized flux of atomic hydrogen
ss
dislocations, grain boundaries, inclusions, and precipitate par-
L = specimen thickness (cm)
ticlesmayactastrapsitesforhydrogenthusdelayinghydrogen
t = time elapsed from commencement of hydrogen
transport.Thesetrapsmaybereversibleorirreversibledepend-
charging (s)
ing on the binding energy associated with the particular trap
t = elapsed time measured extrapolating the linear
b
sites compared to the energy associated with migration for
portionoftherisingpermeationcurrenttransientto
hydrogen in the metal lattice.
J(t)=O(s)
t = time to achieve a value of J(t)/J = 0.63 (s)
lag ss
4.9 The rate of hydrogen atom transport through the metal
x = distance into specimen from the charging surface
duringthefirstpermeationmaybeaffectedbybothirreversible
measured in the thickness direction (cm ).
and reversible trapping as well as by the reduction of any
τ = normalized time (D t/L )
oxidespresentonthechargingsurface.Atsteadystateallofthe
τ = Normalized time to achieve a value of j(t)/J =
lag ss
irreversible traps are occupied. If the mobile hydrogen atoms
0.63 (s)
are then removed and a subsequent permeation test conducted
G148 − 97 (2018)
on the specimen the difference between the first and second
permeation transients can be used to evaluate the influence of
irreversibletrappingontransport,assuminganegligibleroleof
oxide reduction.
4.10 Forsomeenvironments,theconditionsonthecharging
side of the specimen may be suitably altered to induce a decay
of the oxidation current after attainment of steady state. The
rate of decay will be determined by diffusion and reversible
trappingonlyand,hence,canalsobeusedtoevaluatetheeffect
of irreversible trapping on transport during the first transient.
4.11 Comparison of repeated permeation transients with
those obtained for the pure metal can be used in principle to
evaluate the effect of reversible trapping on atomic hydrogen
transport.
4.12 Thispracticeissuitableforsystemsinwhichhydrogen
atomsaregenerateduniformlyoverthechargingsurfaceofthe
membrane. It is not usually applicable for evaluation of
corroding systems in which pitting attack occurs unless the
chargingcellenvironmentisdesignedtosimulatethelocalized
pitenvironmentandtheentiremetalchargingsurfaceisactive.
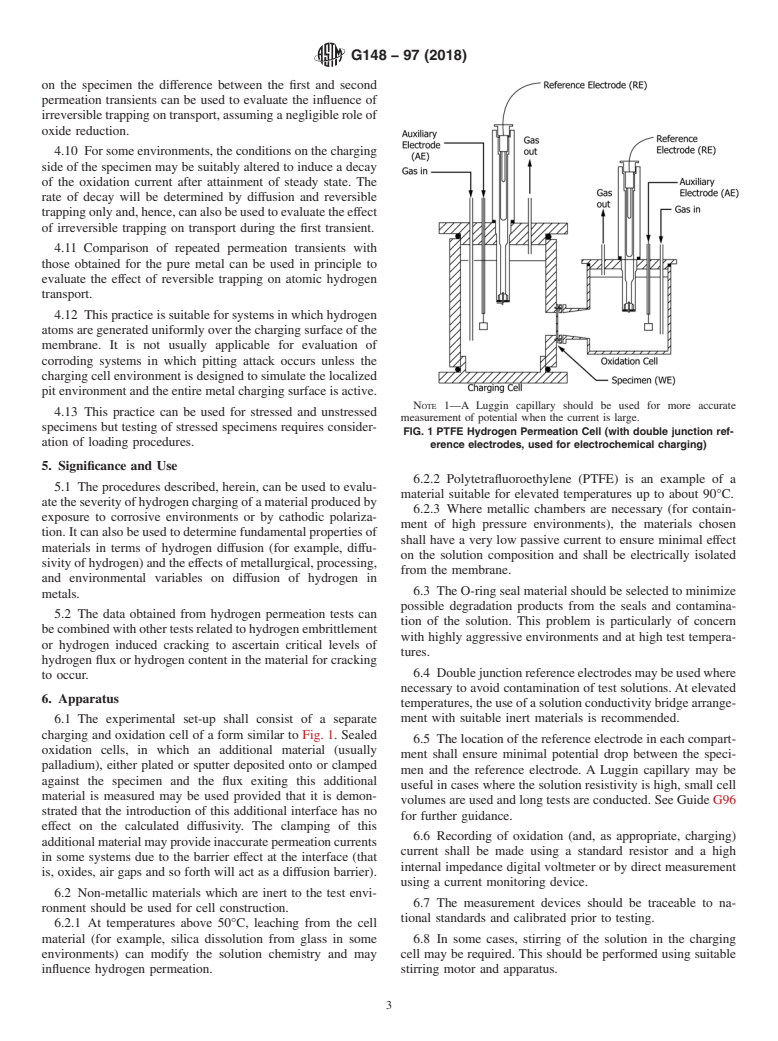
NOTE 1—A Luggin capillary should be used for more accurate
4.13 This practice can be used for stressed and unstressed
measurement of potential when the current is large.
specimens but testing of stressed specimens requires consider-
FIG. 1 PTFE Hydrogen Permeation Cell (with double junction ref-
ation of loading procedures.
erence electrodes, used for electrochemical charging)
5. Significance and Use
6.2.2 Polytetrafluoroethylene (PTFE) is an example of a
5.1 The procedures described, herein, can be used to evalu-
material suitable for elevated temperatures up to about 90°C.
atetheseverityofhydrogenchargingofamaterialproducedby
6.2.3 Where metallic chambers are necessary (for contain-
exposure to corrosive environments or by cathodic polariza-
ment of high pressure environments), the materials chosen
tion.Itcanalsobeusedtodeterminefundamentalpropertiesof
shall have a very low passive current to ensure minimal effect
materials in terms of hydrogen diffusion (for example, diffu-
on the solution composition and shall be electrically isolated
sivityofhydrogen)andtheeffectsofmetallurgical,processing,
from the membrane.
and environmental variables on diffusion of hydrogen in
6.3 TheO-ringsealmaterialshouldbeselectedtominimize
metals.
possible degradation products from the seals and contamina-
5.2 The data obtained from hydrogen permeation tests can
tion of the solution. This problem is particularly of concern
becombinedwithothertestsrelatedtohydrogenembrittlement
with highly aggressive environments and at high test tempera-
or hydrogen induced cracking to ascertain critical levels of
tures.
hydrogen flux or hydrogen content in the material for cracking
6.4 Doublejunctionreferenceelectrodesmaybeusedwhere
to occur.
necessary to avoid contamination of test solutions.At elevated
6. Apparatus
temperatures,theuseofasolutionconductivitybridgearrange-
ment with suitable inert materials is recommended.
6.1 The experimental set-up shall consist of a separate
charging and oxidation cell of a form similar to Fig. 1. Sealed
6.5 Thelocationofthereferenceelectrodeineachcompart-
oxidation cells, in which an additional material (usually
ment shall ensure minimal potential drop between the speci-
palladium), either plated or sputter deposited onto or clamped
men and the reference electrode. A Luggin capillary may be
against the specimen and the flux exiting this additional
useful in cases where the solution resistivity is high, small cell
material is measured may be used provided that it is demon-
volumesareusedandlongtestsareconducted.SeeGuideG96
strated that the introduction of this additional interface has no
for further guidance.
effect on the calculated diffusivity. The clamping of this
6.6 Recording of oxidation (and, as appropriate, charging)
additionalmaterialmayprovideinaccuratepermeationcurrents
current shall be made using a standard resistor and a high
in some systems due to the barrier effect at the interface (that
internal impedance digital voltmeter or by direct measurement
is, oxides, air gaps and so forth will act as a diffusion barrier).
using a current monitoring device.
6.2 Non-metallic materials which are inert to the test
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.