ASTM E2898-13
(Guide)Standard Guide for Risk-Based Validation of Analytical Methods for PAT Applications
Standard Guide for Risk-Based Validation of Analytical Methods for PAT Applications
SIGNIFICANCE AND USE
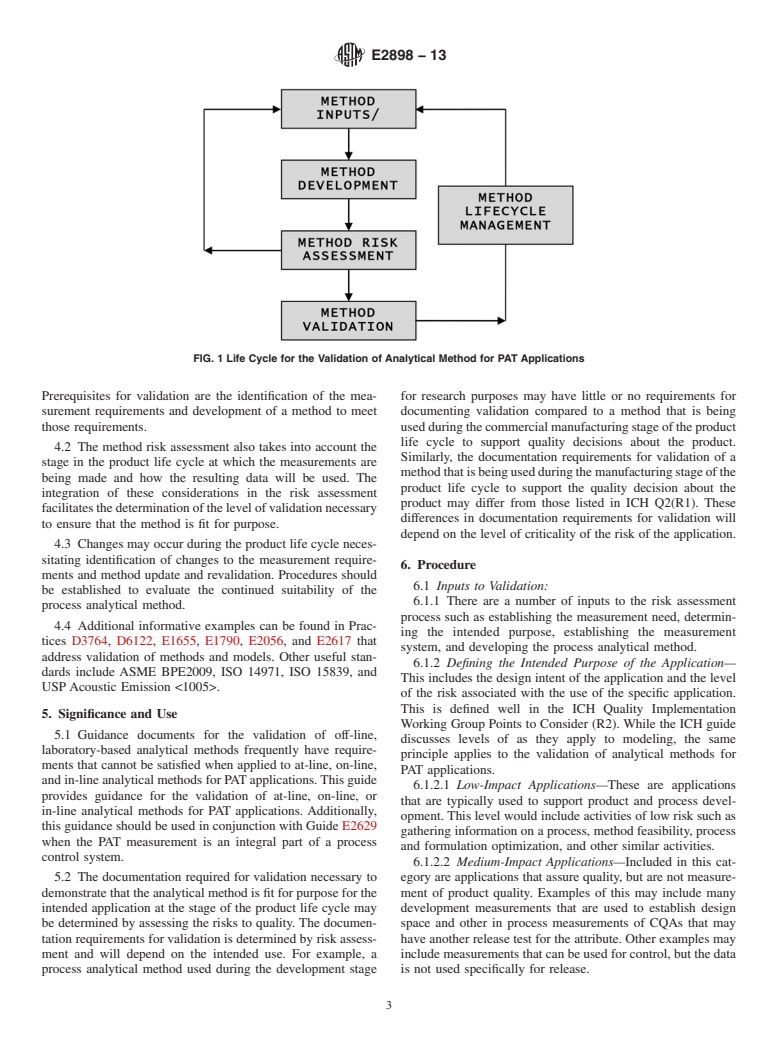
4.1 This guide supports the principles of Guide E2500 and extends these principles to validation of analytical methods for PAT applications. The ongoing process of method validation is graphically represented in Fig. 1, which shows the life cycle of the validation of analytical methods for PAT applications. Prerequisites for validation are the identification of the measurement requirements and development of a method to meet those requirements.
4.2 The method risk assessment also takes into account the stage in the product life cycle at which the measurements are being made and how the resulting data will be used. The integration of these considerations in the risk assessment facilitates the determination of the level of validation necessary to ensure that the method is fit for purpose.
4.3 Changes may occur during the product life cycle necessitating identification of changes to the measurement requirements and method update and revalidation. Procedures should be established to evaluate the continued suitability of the process analytical method.
4.4 Additional informative examples can be found in Practices D3764, D6122, E1655, E1790, E2056, and E2617 that address validation of methods and models. Other useful standards include ASME BPE2009, ISO 14971, ISO 15839, and USP Acoustic Emission .
SCOPE
1.1 This guide provides an overview to the risk-based validation of process analytical methods under a process analytical technology (PAT) paradigm for pharmaceuticals and biopharmaceuticals and as such includes guidance on assessing risk to product quality from inappropriate method validation.
1.2 This guide builds on existing standards on the topic of validation concentrating on applying such standards to analytical methods for on-line analysis. In particular, it addresses the validation of at-line, on-line, or in-line PAT measurements and covers both API and Drug Product (DP) measurements.
1.3 The definitions of International Conference on Harmonization (ICH) validation parameters (such as specificity, precision, repeatability, etc.) apply; however, the method of demonstrating the validation parameters may vary from that described in ICH and is discussed.
1.4 As consistent with the U.S. Food and Drug Administration (FDA) process validation guidance, this document also briefly covers ongoing assurance that the method remains in a validated state during routine use.
1.5 Equipment and instrument qualification are out of the scope of this guide but will be referenced as inputs to validation of analytical methods for PAT applications.
1.6 The validation of multivariate prediction models is out of scope but will be referenced as inputs to validation of analytical methods for PAT applications.
1.7 Microbiological methods are out of scope.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2898 − 13
StandardGuide for
Risk-Based Validation of Analytical Methods for PAT
Applications
This standard is issued under the fixed designation E2898; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This guide provides an overview to the risk-based 2.1 ASTM Standards:
validation of process analytical methods under a process
D3764 Practice forValidation of the Performance of Process
analytical technology (PAT) paradigm for pharmaceuticals and
Stream Analyzer Systems
biopharmaceuticalsandassuchincludesguidanceonassessing D6122 Practice for Validation of the Performance of Multi-
risk to product quality from inappropriate method validation.
variate Online,At-Line, and Laboratory Infrared Spectro-
photometer Based Analyzer Systems
1.2 This guide builds on existing standards on the topic of
E1655 Practices for Infrared Multivariate Quantitative
validation concentrating on applying such standards to analyti-
Analysis
cal methods for on-line analysis. In particular, it addresses the
E1790 Practice for Near Infrared Qualitative Analysis
validation of at-line, on-line, or in-line PAT measurements and
E2056 Practice for Qualifying Spectrometers and Spectro-
covers both API and Drug Product (DP) measurements.
photometers for Use in Multivariate Analyses, Calibrated
1.3 The definitions of International Conference on Harmo-
Using Surrogate Mixtures
nization (ICH) validation parameters (such as specificity,
E2476 Guide for Risk Assessment and Risk Control as it
precision, repeatability, etc.) apply; however, the method of
Impacts the Design, Development, and Operation of PAT
demonstrating the validation parameters may vary from that
Processes for Pharmaceutical Manufacture
described in ICH and is discussed.
E2500 Guide for Specification, Design, and Verification of
Pharmaceutical and Biopharmaceutical Manufacturing
1.4 As consistent with the U.S. Food and DrugAdministra-
Systems and Equipment
tion (FDA) process validation guidance, this document also
E2617 Practice for Validation of Empirically Derived Mul-
briefly covers ongoing assurance that the method remains in a
tivariate Calibrations
validated state during routine use.
E2629 Guide for Verification of ProcessAnalytical Technol-
1.5 Equipment and instrument qualification are out of the
ogy (PAT) Enabled Control Systems
scope of this guide but will be referenced as inputs to
2.2 ICH Standards:
validation of analytical methods for PAT applications.
Q2(R1) Guidance on Validation of Analytical Procedures:
1.6 The validation of multivariate prediction models is out
Text and Methodology
of scope but will be referenced as inputs to validation of
Q7 Good Manufacturing Practice Guide for Active Pharma-
analytical methods for PAT applications.
ceutical Ingredients
Q9 Quality Risk
1.7 Microbiological methods are out of scope.
ICH Quality Implementation Working Group Points to
1.8 This standard does not purport to address all of the
Consider (R2) ICH-Endorsed Guide for ICH Q8/Q9/Q10
safety concerns, if any, associated with its use. It is the
Implementation dated 6 December 2011
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture the ASTM website.
of Pharmaceutical Products and is the direct responsibility of Subcommittee E55.01 Available from International Conference on Harmonisation of Technical
on PAT System Management, Implementation and Practice. Requirements for Registration of Pharmaceuticals for Human Use (ICH), ICH
Current edition approved Nov. 1, 2013. Published December 2013. DOI: Secretariat, c/o IFPMA, 15 ch. Louis-Dunant, P.O. Box 195, 1211 Geneva 20,
10.1520/E2898-13. Switzerland, http://www.ich.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2898 − 13
2.3 Other Standards: 3.1.10.1 Discussion—Qualification is part of validation, but
ASME BPE2009 BioProcessing Equipment Standard the individual qualification steps alone do not constitute
FDA Guidance for Industry Process Validation: General process validation. FDA/ICH Q7A
Principles and Practices
3.1.11 qualitative, adj—type of method whereby a classifi-
ISO 14971 Medical Devices—Application of Risk Manage-
cation (such as pass/fail) is generated for the attribute or
ment to Medical Devices
parameter measured.
ISO 15839 Water Quality—On-line Sensors/Analysing
3.1.11.1 Discussion—The method output may be descrip-
Equipment for Water—Specifications and Performance
tive rather than numerical.
Tests
3.1.12 quantitative, adj—type of method whereby a numeri-
ISO/IEC Guide 51 Safety Aspects—Guidelines for Their
6 cal value or result is generated for the attribute or parameter
Inclusion in Standards
measured.
USP Acoustic Emission <1005>
3.1.13 reference sample, n—substanceofestablishedquality
3. Terminology
used as a reference standard for the method validation.
3.1 Definitions: 3.1.13.1 Discussion—The reference sample may be a refer-
3.1.1 acceptance criteria, n—criteria that a system or com- ence standard (primary or secondary) and may be commercial
ponentshallsatisfytobeacceptedbyauserorotherauthorized or development material for which the value of its relevant
entity. parameter or attribute has been established. E1655
3.1.2 at-line measurements, n—measurement in which the 3.1.14 risk, n—combination of the probability of occurrence
sample is removed, isolated from, and analyzed in close
of harm and the severity of that harm. ISO/IEC Guide 51,
proximity to the process stream. ICH Q9
3.1.3 categorical data, n—measurement output that has
3.1.15 risk analysis, n—the estimation of the risk associated
distinct and predetermined output options (for example, pass/
with the identified hazard. ICH Q9
fail, 1/0, red/yellow/green, and on/off) and is typically nonnu-
3.1.16 risk assessment, n—a systematic process of organiz-
meric in nature.
ing information to support a risk decision to be made within a
3.1.4 continuous data, n—numerical information or output
risk management process. Consisting of identification hazards
having any values within a given range.
and the analysis and evaluation of risks associated with
exposure to those hazards. ICH Q9, ISO 14971
3.1.5 discrete data, n—numerical information for which a
limited set of values are allowed within a given range.
3.1.17 verification, n—systematic approach to demonstrate
that manufacturing systems, acting singly or in combination,
3.1.6 in-line measurements, n—measurement in which the
are fit for intended use, have been properly installed, and are
sample is not removed from the process stream, which may be
operating correctly.
either invasive or noninvasive.
3.1.7 off-line measurements, n—measurement in which the
3.1.17.1 Discussion—This is an umbrella term that encom-
sample is removed, isolated from, and analyzed in an area
passes all types of approaches to assuring systems are fit for
remote from the manufacturing process.
use such as qualification, commissioning and qualification,
verification, system validation, or other validation. There is
3.1.8 on-line measurements, n—measurement in which the
sample is diverted from the manufacturing process and may be recognition that the word verification is used in conjunction
with validating process systems and that the word validation is
returned to the process stream.
used for analytical methods.
3.1.9 process analytical technology (PAT) application,
3.2 Acronyms:
n—the installation/utilization of a measurement system, for
3.2.1 ICH—International Conference on Harmonization of
designing, analyzing, and controlling manufacturing through
Technical Requirements for Registration of Pharmaceuticals
timely measurements (that is, during processing) of critical
for Human Use
quality and performance attributes of raw and in-process
materials and processes, with the goal of ensuring final product 3.2.2 LOD—limit of detection
quality.
3.2.3 LOQ—limit of quantification
3.1.10 qualification, n—action of proving and documenting
3.2.4 PAT—process analytical technology
that equipment or ancillary systems are properly installed,
3.2.5 RTRT —real time release testing
work correctly, and are fit for their intended purpose.
3.2.6 DOE—design of experiments
Available from American Society of Mechanical Engineers (ASME), ASME
International Headquarters, Two Park Ave., New York, NY 10016-5990, http://
4. Significance and Use
www.asme.org.
4.1 This guide supports the principles of Guide E2500 and
Available from Food and Drug Administration (FDA), 10903 New Hampshire
Ave., Silver Spring, MD 20993-0002, http://www.fda.gov.
extends these principles to validation of analytical methods for
Available from International Organization for Standardization (ISO), 1, ch. de
PAT applications. The ongoing process of method validation is
la Voie-Creuse, CP 56, CH-1211 Geneva 20, Switzerland, http://www.iso.org.
graphically represented in Fig. 1, which shows the life cycle of
Available from U.S. Pharmacopeia (USP), 12601 Twinbrook Pkwy., Rockville,
MD 20852-1790, http://www.usp.org. the validation of analytical methods for PAT applications.
E2898 − 13
FIG. 1 Life Cycle for the Validation of Analytical Method for PAT Applications
Prerequisites for validation are the identification of the mea- for research purposes may have little or no requirements for
surement requirements and development of a method to meet documenting validation compared to a method that is being
those requirements. usedduringthecommercialmanufacturingstageoftheproduct
life cycle to support quality decisions about the product.
4.2 The method risk assessment also takes into account the
Similarly, the documentation requirements for validation of a
stage in the product life cycle at which the measurements are
methodthatisbeingusedduringthemanufacturingstageofthe
being made and how the resulting data will be used. The
product life cycle to support the quality decision about the
integration of these considerations in the risk assessment
product may differ from those listed in ICH Q2(R1). These
facilitatesthedeterminationofthelevelofvalidationnecessary
differences in documentation requirements for validation will
to ensure that the method is fit for purpose.
depend on the level of criticality of the risk of the application.
4.3 Changes may occur during the product life cycle neces-
sitating identification of changes to the measurement require-
6. Procedure
ments and method update and revalidation. Procedures should
6.1 Inputs to Validation:
be established to evaluate the continued suitability of the
6.1.1 There are a number of inputs to the risk assessment
process analytical method.
process such as establishing the measurement need, determin-
4.4 Additional informative examples can be found in Prac-
ing the intended purpose, establishing the measurement
tices D3764, D6122, E1655, E1790, E2056, and E2617 that
system, and developing the process analytical method.
address validation of methods and models. Other useful stan-
6.1.2 Defining the Intended Purpose of the Application—
dards include ASME BPE2009, ISO 14971, ISO 15839, and
This includes the design intent of the application and the level
USP Acoustic Emission <1005>.
of the risk associated with the use of the specific application.
This is defined well in the ICH Quality Implementation
5. Significance and Use
Working Group Points to Consider (R2). While the ICH guide
5.1 Guidance documents for the validation of off-line,
discusses levels of as they apply to modeling, the same
laboratory-based analytical methods frequently have require-
principle applies to the validation of analytical methods for
ments that cannot be satisfied when applied to at-line, on-line,
PAT applications.
and in-line analytical methods for PATapplications.This guide
6.1.2.1 Low-Impact Applications—These are applications
provides guidance for the validation of at-line, on-line, or
that are typically used to support product and process devel-
in-line analytical methods for PAT applications. Additionally,
opment. This level would include activities of low risk such as
this guidance should be used in conjunction with Guide E2629
gathering information on a process, method feasibility, process
when the PAT measurement is an integral part of a process
and formulation optimization, and other similar activities.
control system.
6.1.2.2 Medium-Impact Applications—Included in this cat-
5.2 The documentation required for validation necessary to egory are applications that assure quality, but are not measure-
demonstrate that the analytical method is fit for purpose for the ment of product quality. Examples of this may include many
intended application at the stage of the product life cycle may development measurements that are used to establish design
be determined by assessing the risks to quality. The documen- space and other in process measurements of CQAs that may
tation requirements for validation is determined by risk assess- have another release test for the attribute. Other examples may
ment and will depend on the intended use. For example, a include measurements that can be used for control, but the data
process analytical method used during the development stage is not used specifically for release.
E2898 − 13
6.1.2.3 High Impact Applications—These are applications information purposes, where as much more extensive docu-
that fall into the Real Time Release Testing (RTRT) category. mentation may be required to demonstrate validation of high
This is the application that incorporates the measurement to
level applications during the development stage of the life
insure product quality by control of the process or is a cycle. During the development stage of the product life cycle,
substitute for a specification test such as product assay or is
the impact to product quality will typically come from the fact
replacement for dissolution.
thatmeasurementdatageneratedbythemethodmaybeusedto
6.1.3 Establishing the PAT Measurement System— make decisions concerning the design of the p
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.