ASTM E2629-20
(Guide)Standard Guide for Verification of Process Analytical Technology (PAT) Enabled Control Systems
Standard Guide for Verification of Process Analytical Technology (PAT) Enabled Control Systems
SIGNIFICANCE AND USE
5.1 This guide supports the principles of Guide E2500 and extends these principles to the verification of PAT-enabled control systems.
5.2 This guide clarifies what is important for verification of PAT-enabled control systems. Such systems are often complex and require multidisciplinary and cross-functional teams to achieve optimum results. This guide provides a common basis for understanding requirements for all involved disciplines such as control engineering, development, manufacturing, and process validation.
SCOPE
1.1 This guide describes the verification of process analytical technology (PAT) enabled control systems using a science- and risk-based approach. It establishes principles for determining the scope and extent of verification activities necessary to ensure that the PAT-enabled control system is fit for purpose, properly implemented, and functions as expected.
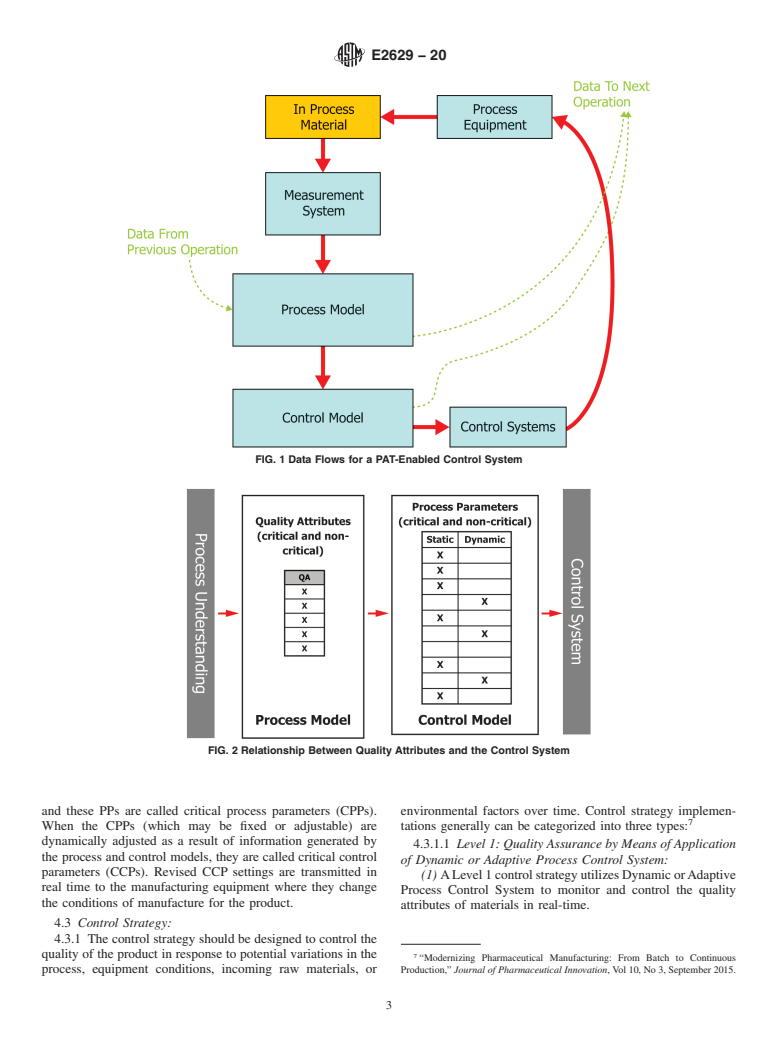
1.2 In this guide, a PAT-enabled control system is considered to be the system that adjusts the manufacturing process using timely measurements (that is, during processing) of attributes of raw and in-process materials to determine responses that assure the process remains within specified boundaries and minimizes variability in the output material. The overall aim of the PAT-enabled control system is to ensure product quality. The PAT-enabled control system of a manufacturing process provides the capability to determine the current status of the process and drive the process to ensure the output material has the desired quality characteristics. The control system should be able to respond to process variations in a timely manner, providing corrections that ensure that the process follows the desired process trajectory to reach the desired outcome. PAT-enabled control systems may use process models based on first principles understanding or empirical models derived from experimental investigations or both. In addition to automated controls, a PAT-enabled control system may include components where there is manual intervention.
1.3 Principles described in this guide may be applied regardless of the complexity or scale of the PAT-enabled control system or whether applied to batch or continuous processing, or both. The intention of this standard is to describe and support the implementation of a PAT enabled Control Strategy, as described in ICH Q8(R2).
1.4 The principles described in this guide are applicable to a PAT-enabled control system and also to its component subsystems. This guide does not cover the requirements for continuous quality verification of the overall process, which are covered in Guide E2537, or for validation of PAT methods, which is covered in Guide E2898.
1.5 For information on science- and risk-based approaches in the pharmaceutical industry, reference should be made to ICH Q8(R2), ICH Q9, and ICH Q10. For guidance on PAT systems in the pharmaceutical industry, reference should be made to FDA Guidance for Industry—PAT and FDA Guidance for Industry—Process Validation, as well as EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use and EU Guideline on Process Validation for Finished Products.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E2629 − 20
Standard Guide for
Verification of Process Analytical Technology (PAT) Enabled
1
Control Systems
This standard is issued under the fixed designation E2629; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope subsystems. This guide does not cover the requirements for
continuous quality verification of the overall process, which
1.1 This guide describes the verification of process analyti-
are covered in Guide E2537, or for validation of PAT methods,
cal technology (PAT) enabled control systems using a science-
which is covered in Guide E2898.
and risk-based approach. It establishes principles for determin-
ing the scope and extent of verification activities necessary to
1.5 For information on science- and risk-based approaches
ensure that the PAT-enabled control system is fit for purpose, in the pharmaceutical industry, reference should be made to
properly implemented, and functions as expected.
ICH Q8(R2), ICH Q9, and ICH Q10. For guidance on PAT
systems in the pharmaceutical industry, reference should be
1.2 In this guide, a PAT-enabled control system is consid-
made to FDA Guidance for Industry—PAT and FDA Guidance
ered to be the system that adjusts the manufacturing process
for Industry—Process Validation, as well as EU Guidelines for
using timely measurements (that is, during processing) of
Good Manufacturing Practice for Medicinal Products for
attributes of raw and in-process materials to determine re-
Human and Veterinary Use and EU Guideline on Process
sponses that assure the process remains within specified
Validation for Finished Products.
boundaries and minimizes variability in the output material.
The overall aim of the PAT-enabled control system is to ensure
1.6 This standard does not purport to address all of the
product quality. The PAT-enabled control system of a manu-
safety concerns, if any, associated with its use. It is the
facturing process provides the capability to determine the
responsibility of the user of this standard to establish appro-
current status of the process and drive the process to ensure the
priate safety, health, and environmental practices and deter-
output material has the desired quality characteristics. The
mine the applicability of regulatory limitations prior to use.
control system should be able to respond to process variations 1.7 This international standard was developed in accor-
in a timely manner, providing corrections that ensure that the
dance with internationally recognized principles on standard-
process follows the desired process trajectory to reach the ization established in the Decision on Principles for the
desired outcome. PAT-enabled control systems may use pro-
Development of International Standards, Guides and Recom-
cess models based on first principles understanding or empiri- mendations issued by the World Trade Organization Technical
cal models derived from experimental investigations or both.
Barriers to Trade (TBT) Committee.
In addition to automated controls, a PAT-enabled control
2. Referenced Documents
system may include components where there is manual inter-
2
vention.
2.1 ASTM Standards:
1.3 Principles described in this guide may be applied E122 Practice for Calculating Sample Size to Estimate, With
regardless of the complexity or scale of the PAT-enabled Specified Precision, the Average for a Characteristic of a
control system or whether applied to batch or continuous Lot or Process
E2363 Terminology Relating to Manufacturing of Pharma-
processing, or both. The intention of this standard is to describe
and support the implementation of a PAT enabled Control ceutical and Biopharmaceutical Products in the Pharma-
ceutical and Biopharmaceutical Industry
Strategy, as described in ICH Q8(R2).
E2476 Guide for Risk Assessment and Risk Control as it
1.4 The principles described in this guide are applicable to
Impacts the Design, Development, and Operation of PAT
a PAT-enabled control system and also to its component
Processes for Pharmaceutical Manufacture
1
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture
of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Subcommittee E55.13 on Process Evaluation and Control.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Current edition approved Aug. 1, 2020. Publ
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E2629 − 19 E2629 − 20
Standard Guide for
Verification of Process Analytical Technology (PAT) Enabled
1
Control Systems
This standard is issued under the fixed designation E2629; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide describes the verification of process analytical technology (PAT) enabled control systems using a science- and
risk-based approach. It establishes principles for determining the scope and extent of verification activities necessary to ensure that
the PAT-enabled control system is fit for purpose, properly implemented, and functions as expected.
1.2 In this guide, a PAT-enabled control system is considered to be the system that adjusts the manufacturing process using
timely measurements (that is, during processing) of attributes of raw and in-process materials to determine responses that assure
the process remains within specified boundaries and minimizes variability in the output material. The overall aim of the
PAT-enabled control system is to ensure product quality. The PAT-enabled control system of a manufacturing process provides the
capability to determine the current status of the process and drive the process to ensure the output material has the desired quality
characteristics. The control system should be able to respond to process variations in a timely manner, providing corrections that
ensure that the process follows the desired process trajectory to reach the desired outcome. PAT-enabled control systems may use
process models based on first principles understanding or empirical models derived from experimental investigations or both. In
addition to automated controls, a PAT-enabled control system may include components where there is manual intervention.
1.3 Principles described in this guide may be applied regardless of the complexity or scale of the PAT-enabled control system
or whether applied to batch or continuous processing, or both. The intention of this standard is to describe and support the
implementation of a PAT enabled Control Strategy, as described in ICH Q8(R2).
1.4 The principles described in this guide are applicable to a PAT-enabled control system and also to its component subsystems.
This guide does not cover the requirements for continuous quality verification of the overall process, which are covered in Guide
E2537, or for validation of PAT methods, which is covered in Guide E2898.
1.5 For information on science- and risk-based approaches in the pharmaceutical industry, reference should be made to
ICH Q8(R2), ICH Q9, and ICH Q10. For guidance on PAT systems in the pharmaceutical industry, reference should be made to
FDA Guidance for Industry—PAT and FDA Guidance for Industry—Process Validation, as well as EU Guidelines for Good
Manufacturing Practice for Medicinal Products for Human and Veterinary Use and EU Guideline on Process Validation for
Finished Products.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E122 Practice for Calculating Sample Size to Estimate, With Specified Precision, the Average for a Characteristic of a Lot or
Process
1
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
Subcommittee E55.01 on Process Understanding and PAT System Management, Implementation and Practice.
Current edition approved June 15, 2019Aug. 1, 2020. Published July 2019August 2020. Originally approved in 2011. Last previous edition approved in 20112019 as E2629
– 11.19. DOI: 10.1520/E2629-19.10.1520/E2629-20.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org.
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.