ASTM G186-05(2011)
(Test Method)Standard Test Method for Determining Whether Gas-Leak-Detector Fluid Solutions Can Cause Stress Corrosion Cracking of Brass Alloys
Standard Test Method for Determining Whether Gas-Leak-Detector Fluid Solutions Can Cause Stress Corrosion Cracking of Brass Alloys
SCOPE
1.1 This test method covers an accelerated test method for evaluating the tendency of gas leak detection fluids (LDFs) to cause stress corrosion cracking (SCC) of brass components in compressed gas service.
1.2 The values stated in inch-pound units are to be regarded as standard. The values given in parentheses are mathematical conversions to SI units that are provided for information only and are not considered standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G186 − 05(Reapproved 2011)
Standard Test Method for
Determining Whether Gas-Leak-Detector Fluid Solutions
Can Cause Stress Corrosion Cracking of Brass Alloys
This standard is issued under the fixed designation G186; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
3.1 Definitions of Terms Specific to This Standard:
1.1 This test method covers an accelerated test method for
3.1.1 Gas Leak Detector Solutions—Also known as leak
evaluating the tendency of gas leak detection fluids (LDFs) to
detection fluids, leak detector solutions, bubble solutions, and
cause stress corrosion cracking (SCC) of brass components in
soap solutions, designated in this standard as LDFs, are fluids
compressed gas service.
used to detect leaks in pressurized gas systems by the forma-
1.2 The values stated in inch-pound units are to be regarded
tion of bubbles at the leak site.
as standard. The values given in parentheses are mathematical
3.1.2 The terminology used herein, if not specifically de-
conversions to SI units that are provided for information only
finedotherwise,shallbeinaccordancewithTerminologyG15.
and are not considered standard.
1.3 This standard does not purport to address all of the
4. Summary of Test Method
safety concerns, if any, associated with its use. It is the
4.1 This test method consists of three steps: The first step
responsibility of the user of this standard to establish appro-
consistsofrunningasampleofthetestspecimenstoverifythat
priate safety and health practices and to determine the
they are susceptible to stress corrosion cracking using Matts-
applicability of regulatory limitations prior to use.
son’sSolution(seePracticeG37).Thesecondstepistoexpose
the specimens to a solution that does not cause SCC to verify
2. Referenced Documents
thatthetestenvironmentdoesnotcontaincomponentsthatcan
cause SCC to brass. The third step is to test the LDF to
2.1 ASTM Standards:
determine if it causes SCC of the brass specimens within 15
B135Specification for Seamless Brass Tube
wetting and evaporation cycles.
B135MSpecification for Seamless Brass Tube [Metric]
D1193Specification for Reagent Water
4.2 The specimen used in this test is a C-ring stressed to
G1Practice for Preparing, Cleaning, and Evaluating Corro-
create at least 0.65% strain in the outer fibers of the specimen.
sion Test Specimens
4.3 Macroscopic examination of the specimens is carried
G15TerminologyRelatingtoCorrosionandCorrosionTest-
out after every second wetting cycle and if cracking is
ing (Withdrawn 2010)
suspected the specimen is examined at higher magnifications
G37Practice for Use of Mattsson’s Solution of pH 7.2 to
for confirmation. Metallographic sectioning through the
Evaluate the Stress-Corrosion Cracking Susceptibility of
stressed area is used to verify minor cracking at the end of the
Copper-Zinc Alloys
fifteen cycles.
G38 Practice for Making and Using C-Ring Stress-
4.4 LDFs that cause SCC in any specimens within 15
Corrosion Test Specimens
wetting cycles are considered to have failed this test and not
suitable for use in pressurized gas systems with brass compo-
nents.
This test method is under the jurisdiction of ASTM Committee G01 on
Corrosion of Metals and is the direct responsibility of Subcommittee G01.06 on
5. Significance and Use
Environmentally Assisted Cracking.
Current edition approved March 1, 2011. Published April 2011. Originally
5.1 Brass components are routinely used in compressed gas
approved in 2005. Last previous edition approved in 2005 as G186–05. DOI:
service for valves, pressure regulators, connectors and many
10.1520/G0186-05R11.
other components. Although soft brass is not susceptible to
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
ammonia SCC, work-hardened brass is susceptible if its
Standards volume information, refer to the standard’s Document Summary page on
hardnessexceedsabout54HR30T(55HRB)(Rockwellscale).
the ASTM website.
Normal assembly of brass components should not induce
The last approved version of this historical standard is referenced on
www.astm.org. sufficient work hardening to cause susceptibility to ammonia
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G186 − 05 (2011)
SCC. However, it is has been observed that over-tightening of committee on Analytical Reagents of the American Chemical
the components will render them susceptible to SCC, and the Society, where such specifications are applicable, shall be
problem becomes more severe in older components that have used.
been tightened many times. In this test, the specimens are
7.2 Finepurecopperpowderwithparticlesize<68µmshall
obtainedinthehardenedconditionandarestrainedbeyondthe
be used.
elastic limit to accelerate the tendency towards SCC.
7.3 Solutionsusingwatershallbepreparedusingdistilledor
5.2 It is normal practice to use LDFs to check pressurized
deionized water conforming to the purity requirements of
systems to assure that leaking is not occurring. LDFs are
Specification D1193, Type IV reagent water.
usually aqueous solutions containing surfactants that will form
7.4 Leakdetectorsolutionsshallmeetmanufacturer’sspeci-
bubbles at the site of a leak. If the LDF contains ammonia or
fications.
other agent that can cause SCC in brass, serious damage can
occur to the system that will compromise its safety and
7.5 Mattsson’s Solution shall be freshly prepared according
integrity.
to Practice G37.
5.3 It is important to test LDFs to assure that they do not
causeSCCofbrassandtoassurethattheuseoftheseproducts 8. Hazards
does not compromise the integrity of the pressure containing
8.1 Consult Material Safety Data Sheets (MSDS) for all
system.
chemicals both reagent and commercial before testing to gain
5.4 It has been found that corrosion of brass is necessary
a full understanding of any potential hazards.
before SCC can occur.The reason for this is that the corrosion
8.2 The test solutions present no undue safety hazard. It is
process results in cupric and cuprous ions accumulating in the
recommended, however, that appropriate personnel protection
electrolyte. Therefore, adding copper metal and cuprous oxide
equipment such as resistant gloves and shatterproof eyewear
(Cu O) to the aqueous solution accelerates the SCC process if
withsideshieldsbewornwhenthechemicalsorspecimensare
agents that cause SCC are present. However, adding these
handled.
components to a solution that does not cause SCC will not
8.3 The solutions contain copper and are thus considered
make stressed brass crack.
poisonous so they should not be ingested.
5.5 Repeated application of the solution to the specimen
8.4 Ammonium sulfate, (NH ) SO , in the Mattsson’s solu-
followed by a drying period causes the components in the
4 2 4
tion has been reported to be allergenic. Repeated short-time
solution to concentrate thereby further increasing the rate of
skin contact with the solution over extended periods of time
cracking. This also simulates service where a system may be
tested many times during its life. These features of the test should be avoided.
method accelerate the test and allow an answer to be obtained
8.5 The fumes given off by the Mattsson’s test solution
more rapidly.
contain ammonia. The least detectable ammonia odor corre-
5.6 This test method applies only to brasses. Successful
spondstoaconcentrationof50ppm;100ppmcanbetolerated
passage of this test does not assure that the LDF will be for several hours without serious disturbance; 700 ppm causes
acceptable for use on other alloy systems such as stainless immediate eye irritation; and greater than 5000 ppm can be
steels or aluminum alloys. lethal. The mixing and the actual testing with Mattsson’s
solution should therefore be run in a well-ventilated area.
6. Interferences
6.1 When conducting this test, it is very important that the
9. Test Solutions
air not be contaminated with ammonia vapors. Reagent bottles
9.1 Control Solution (benign water solution)—Add2.5gCu
withammoniumhydroxideorotherteststhatinvolveammonia
powder and 2.5 g Cu O to 1000 mLof H O. Then add 10 mL
2 2
or its compounds including amines must not be in the vicinity
of glycerin to solution. Shake solution vigorously to thor-
of these tests. This also includes Mattsson’s test solution.
oughly mix contents.
6.2 Cross contamination may result in false stress corrosion
9.2 Leak Detector Solutions—Add 2.5 g Cu powder and
cracking results therefore concurrent exposure tests with dif-
2.5g Cu O to 1000 mL of each manufacturer’s solution to be
ferent leak detector solutions should be conducted in such a
tested. Shake solutions vigorously to thoroughly mix contents.
way that any set of samples does not influence the results of
any of the other samples.
NOTE 1—Some of the solids will settle out of the solutions in between
cycles, therefore, it is very important to shake the solutions vigorously
6.3 In this test, the susceptibility of the C-ring specimens to
prior to their use in the wetting cycle.
crack in a particular test solution can be affected by the temper
of the brass alloy; therefore, it is crucial that the C-rings be
fabricated from hard drawn temper brass tubing that meets the
Reagent Chemicals, American Chemical Society Specifications, American
minimum specified hardness requirements.
Chemical Society, Washington, DC. For Suggestions on the testing of reagents not
listed by the American Chemical Society, see Annual Standards for Laboratory
7. Reagents and Materials
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
7.1 Reagent grade cuprous oxide (Cu O) and USP/FCC
2 and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
grade glycerin (C H O ) conforming to specifications of the MD.
3 8 3
G186 − 05 (2011)
NOTE2—Everyprecautionshallbetakentomaintaintheintegrityofthe
10. Test Specimen
surfaceafterthefinalpreparation.Avoidroughhandlingthatcouldmarthe
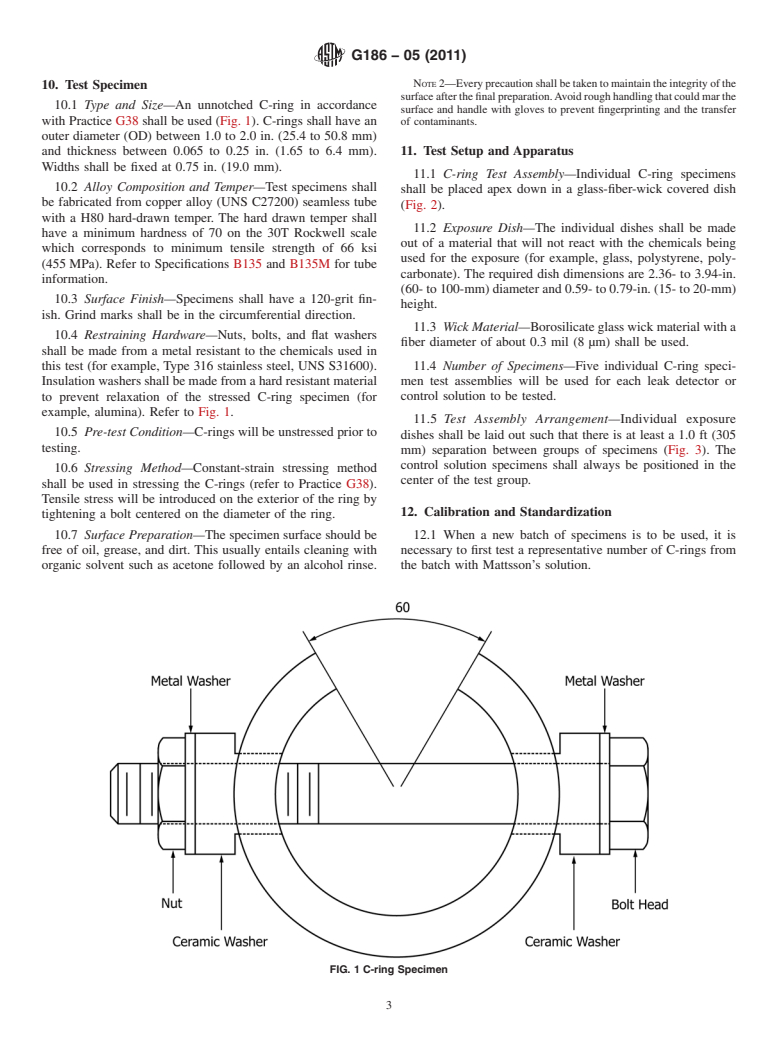
10.1 Type and Size—An unnotched C-ring in accordance
surface and handle with gloves to prevent fingerprinting and the transfer
with Practice G38 shall be used (Fig. 1). C-rings shall have an
of contaminants.
outer diameter (OD) between 1.0 to 2.0 in. (25.4 to 50.8 mm)
and thickness between 0.065 to 0.25 in. (1.65 to 6.4 mm). 11. Test Setup and Apparatus
Widths shall be fixed at 0.75 in. (19.0 mm).
11.1 C-ring Test Assembly—Individual C-ring specimens
10.2 Alloy Composition and Temper—Test specimens shall
shall be placed apex down in a glass-fiber-wick covered dish
be fabricated from copper alloy (UNS C27200) seamless tube
(Fig. 2).
with a H80 hard-drawn temper. The hard drawn temper shall
11.2 Exposure Dish—The individual dishes shall be made
have a minimum hardness of 70 on the 30T Rockwell scale
out of a material that will not react with the chemicals being
which corresponds to minimum tensile strength of 66 ksi
used for the exposure (for example, glass, polystyrene, poly-
(455MPa). Refer to Specifications B135 and B135M for tube
carbonate). The required dish dimensions are 2.36- to 3.94-in.
information.
(60-to100-mm)diameterand0.59-to0.79-in.(15-to20-mm)
10.3 Surface Finish—Specimens shall have a 120-grit fin-
height.
ish. Grind marks shall be in the circumferential direction.
11.3 Wick Material—Borosilicateglasswickmaterialwitha
10.4 Restraining Hardware—Nuts, bolts, and flat washers
fiber diameter of about 0.3 mil (8 µm) shall be used.
shall be made from a metal resistant to the chemicals used in
this test (for example, Type 316 stainless steel, UNS S31600). 11.4 Number of Specimens—Five individual C-ring speci-
Insulationwashersshallbemadefromahardresistantmaterial men test assemblies will be used for each leak detector or
to prevent relaxation of the stressed C-ring specimen (for control solution to be tested.
example, alumina). Refer to Fig. 1.
11.5 Test Assembly Arrangement—Individual exposure
10.5 Pre-test Condition—C-rings will be unstressed prior to
dishes shall be laid out such that there is at least a 1.0 ft (305
testing.
mm) separation between groups of specimens (Fig. 3). The
control solution specimens shall always be positioned in the
10.6 Stressing Method—Constant-strain stressing method
center of the test group.
shall be used in stressing the C-rings (refer to Practice G38).
Tensile stress will be introduced on the exterior of the ring by
12. Calibration and Standardization
tightening a bolt centered on the diameter of the ring.
10.7 Surface Preparation—The specimen surface should be 12.1 When a new batch of specimens is to be used, it is
free of oil, grease, and dirt. This usually entails cleaning with necessary to first test a representative number of C-rings from
organic solvent such as acetone followed by an alcohol rinse. the batch with Mattsson’s solution.
FIG. 1 C-ring Specimen
G186 − 05 (2011)
FIG. 2 C-ring Specimen Ready for Exposure to Test Solution
FIG. 3 Arrangement and Spacing of Specimens for Multiple Solution Testing
12.2 Mattsson’s Solution test must produce cracking of the
test specimens before any further testing is carried out.
G186 − 05 (2011)
TABLE 1 Deflection for Stressing C-rings
12.3 If cracking is not produced during the predescribed
Mattsson’s solution test the following variables need to be O.D. (inch) Wall Thickness (in.)
verified before rejecting the batch of C-rings. 0.065 0.08 0.091 0.125 0.25
12.3.1 Specimen material hardness shall exceed 70 HR 30T
1 0.073 0.059 0.051 0.036 0.015
(Rockwell scale). 1
1 ⁄16 0.083 0.067 0.058 0.041 0.018
1 ⁄8 0.094 0.075 0.065 0.046 0.020
12.3.2 Strain should be checked with a strain gauge if
1 ⁄16 0.105 0.089 0.073 0.052 0.023
hardness is sufficient.
1 ⁄4 0.116 0.083 0.081 0.057 0.026
12.3.3 Mattsson’s solution should be checked to make sure
1 ⁄16 0.129 0.099 0.090 0.064 0.028
1 ⁄8 0.142 0.114 0.099 0.070 0.032
it conforms to Practice G37.
1 ⁄2 0.169 0.136 0.119 0.084 0.038
1 ⁄16 0.184 0.148 0.129 0.092 0.042
12.4 Follow steps in Annex A1 to test the C-rings in
1 ⁄8 0.199 0.160 0.140 0.100 0.046
Mattsson’s Solution.
1 ⁄4 0.232 0.187 0.163 0.116 0.054
1 ⁄8 0.267 0.215 0.188 0.134 0.062
2 0.304 0.245 0.214 0.153 0.072
13. Air Conditions
13.1 Temperature—Airtemperatureshallbemaintainedina
range of 70 to 80°F (21 to 27°C) through
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.