ASTM F116-00(2009)
(Specification)Standard Specification for Medical Screwdriver Bits
Standard Specification for Medical Screwdriver Bits
ABSTRACT
This specification covers the acceptable material and dimensional requirements, and tolerances for bits of screwdrivers used for inserting and removing metal screws applied in surgical implants. The medical screwdrivers are available in the following types: Type I—single-slot bit; Type II—cruciate-slot bit; Type III—cross-slot (modified Phillips) bit; Type IV—hexagonal bit; Type V—square bit; Type VI—hexalobe bit. The bit and shaft portion should be fabricated from either martensitic stainless steel or cold worked cobalt-chromium-tungsten-nickel alloy, as specified. The portions should also meet specified values of Rockwell hardness.
SCOPE
1.1 This specification covers the acceptable dimensions and tolerances for bits of screwdrivers to insert and remove metal screws used as surgical implants.
1.2 This specification is based, in part, upon ISO 8319–1 and ISO 8319–2.
1.3 The screwdrivers with the bits described in this specification are suitable for use with screws described in Specification F 543, ISO 5835, and ISO 9268.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F116 −00(Reapproved 2009)
Standard Specification for
1
Medical Screwdriver Bits
ThisstandardisissuedunderthefixeddesignationF116;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
3
1. Scope 2.2 ISO Standards:
ISO 5832–5 Implants for Surgery—Metallic Materials—
1.1 This specification covers the acceptable dimensions and
Part 5: Wrought Cobalt-Chromium-Tungsten-Nickel Al-
tolerances for bits of screwdrivers to insert and remove metal
loy
screws used as surgical implants.
ISO 5835 Implants for Surgery—Metal Bone Screws with
1.2 This specification is based, in part, upon ISO 8319–1
HexagonalDriverConnection,SphericalUnderSurfaceof
and ISO 8319–2.
Head, Asymmetrical Thread—Dimensions
ISO 7153–1 Surgical Instruments—Metallic Materials—
1.3 The screwdrivers with the bits described in this speci-
fication are suitable for use with screws described in Specifi- Part 1: Stainless Steel
ISO 8319–1 Orthopaedic Instruments—Drive Connections
cation F543, ISO 5835, and ISO 9268.
—Part 1: Keys for Use with Screws with Hexagon Socket
1.4 The values stated in SI units are to be regarded as
Heads
standard. No other units of measurement are included in this
ISO 8319–2 Orthopaedic Instruments—Drive Connections
standard.
—Part 2: Screwdrivers for Single Slot Head Screws,
1.5 This standard does not purport to address all of the
screws with Cruciate Slot, and Cross-Recessed Head
safety concerns, if any, associated with its use. It is the
Screws
responsibility of the user of this standard to establish appro-
ISO 9268 Implants for Surgery—Metal Bone Screws with
priate safety and health practices and determine the applica-
Conical Under-Surface of Head—Dimensions
bility of regulatory limitations prior to use.
3. Classification
2. Referenced Documents
3.1 This specification includes the following types of bits
2
2.1 ASTM Standards:
for medical screwdrivers:
E18 Test Methods for Rockwell Hardness of Metallic Ma-
3.1.1 Type I—Single-slot bit.
terials
3.1.2 Type II—Cruciate-slot bit.
F90 Specification for Wrought Cobalt-20Chromium-
3.1.3 Type III—Cross-slot (Modified Phillips) bit.
15Tungsten-10NickelAlloy for Surgical ImplantApplica-
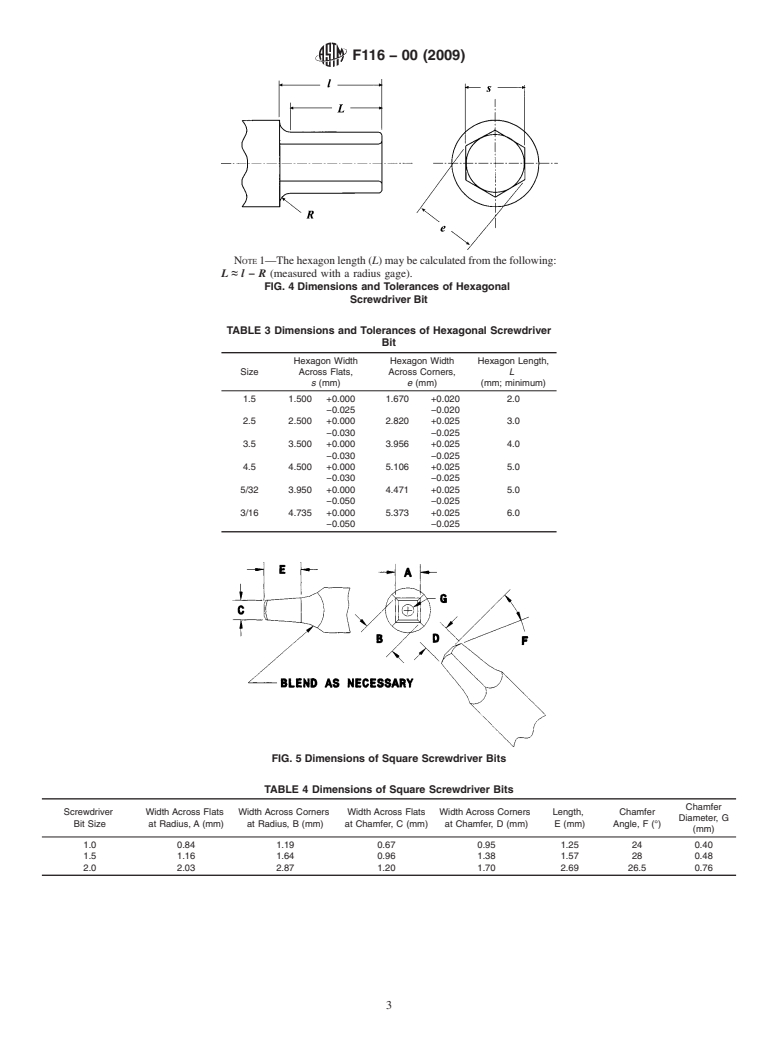
3.1.4 Type IV—Hexagonal bit.
tions (UNS R30605)
3.1.5 Type V—Square bit.
F543 Specification and Test Methods for Metallic Medical
3.1.6 Type VI—Hexalobe bit.
Bone Screws
F565 PracticeforCareandHandlingofOrthopedicImplants
4. Dimensions and Tolerances
and Instruments
F899 Specification for Wrought Stainless Steels for Surgical
4.1 Screwdriver bits conforming to this specification shall
Instruments
be fabricated in accordance with the dimensions and tolerances
F1744 Guide for Care and Handling of Stainless Steel
described below:
Surgical Instruments
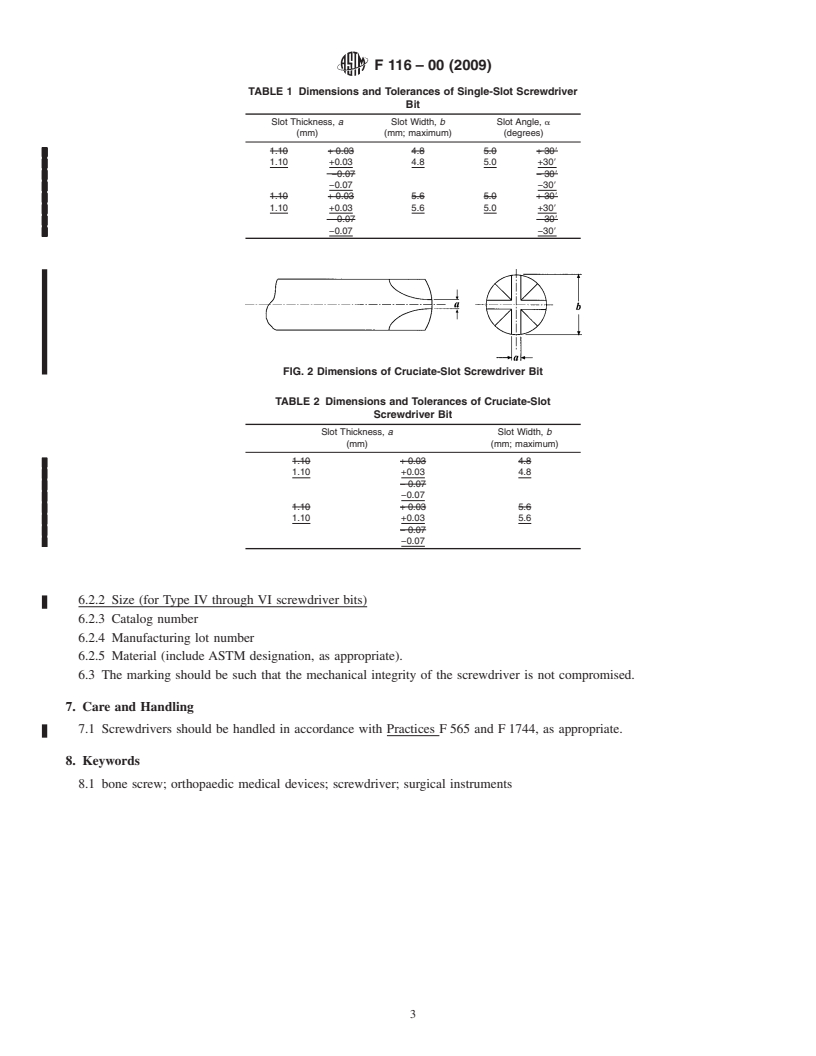
4.1.1 Type I—Single-slot screwdriver bits must conform to
the dimensions and tolerances provided in Table 1, and
1 described in Fig. 1.
This specification is under the jurisdiction of ASTM Committee F04 on
Medical and Surgical Materials and Devices and is the direct responsibility of
4.1.2 Type II—Cruciate-slot screwdriver bits must conform
Subcommittee F04.21 on Osteosynthesis.
to the dimensions and tolerances provided in Table 2, and
Current edition approved April 1, 2009. Published April 2009. Originally
described in Fig. 2.
approvedin1969.Lastpreviouseditionapprovedin2004asF116 – 00(2004).DOI:
10.1520/F0116-00R09.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F116−00 (2009)
FIG. 1Dimensions of Single-Slot Screwdriver Bit
TABLE 1 Dimensions and Tolerances of Single-Slot Screwdriver
Bit
Slot Thickness, a Slot Width, b Slot Angle, a
(mm) (mm; maximum) (degrees)
1.10 +0.03 4.8 5.0 +30'
−0.07 −30'
1.10 +0.03 5.6 5.0 +30'
−0.07 −30'
FIG. 3Dimensions and Tolerances of Cross-Slot (Modified Phil-
lips) Screwdriver Bit
5.1.2 Cold worked Cobalt-Chromium-Tungsten-Nickel al-
loy (Specification F90 or ISO 5832–5).
5.2 The hardness of the material of the bit and shaft portion
shall be 45–55 (stainless steel) or 45–50 (Cobalt-Chromium-
FIG. 2Dimensions of Cruciate-Slot Screwdriver Bit
Tungsten-Nickel alloy) when measured on the Rockwell C
scale according to the procedures described in Test
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:F 116–98
Standard Specification for Designation: F 116 – 00
(Reapproved 2009)
Standard Specification for
1
Medical Screwdriver Bits
This standard is issued under the fixed designation F 116; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This specification covers the acceptable dimensions and tolerances for bits of screwdrivers to insert and remove metal
screws used as surgical implants.
1.2This specification is based, in part, upon ISO 8319-1 and ISO 8319-2.
1.3The screwdrivers with the bits described in this standard are suitable for use with screws described in F543, ISO 5835, and
ISO 9268.
1.4This standard may involve the use of hazardous materials, operations, and equipment. This standard does not purport to
address all of the safety concerns associated with its use. It is the responsibility of whoever uses this standard to consult and
establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
1.2 This specification is based, in part, upon ISO 8319–1 and ISO 8319–2.
1.3 The screwdrivers with the bits described in this specification are suitable for use with screws described in Specification
F 543, ISO 5835, and ISO 9268.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
E18 Standard Test Methods for Rockwell Hardness and Rockwell Superficial Hardness of Metallic MaterialsTest Methods for
Rockwell Hardness of Metallic Materials
F90 Standard Specification for Wrought Cobalt-20Chromium-15Tungsten-10Nickel Alloy for Surgical Implant Applications
(UNS R30605)
F 543 Standard Specification and Test Methods for Metallic Medical Bone Screws
F 565 Standard Practice for Care and Handling of Orthopaedic Implants and Instruments
3
F 899 Standard Specification for Stainless Steel Billet, Bar, and Wire for Surgical Instruments Specification for Wrought
Stainless Steels for Surgical Instruments
F 1744 Standard Practice Guide for Care and Handling of Stainless Steel Surgical Instruments
3
2.2 ISO Standards:
5832-5Implants ISO 5832–5 Implants for Surgery—Metallic Materials—Part 5: Wrought Cobalt-Chromium-Tungsten-Nickel
Alloy
1
This specification is under the jurisdiction ofASTM Committee F-4 F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.21 on Osteosynthesis.
Current edition approved June 10, 1998. Published September 1998. Originally published as F116–69. Last previous edition F116–85 (1991).
Current edition approved April 1, 2009. Published April 2009. Originally approved in 1969. Last previous edition approved in 2004 as F 116 – 00(2004).
2
Annual Book of ASTM Standards, Vol 03.01.
2
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book ofASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Annual Book of ASTM Standards, Vol 13.01.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
F 116 – 00 (2009)
ISO 5835 Implants for Surgery—Metal Bone Screws with Hexagonal Driver Connection, Spherical Under Surface of Head,
Asymmetrical Thread—Dimensions
7153-1 ISO 7153–1 Surgical Instruments—Metallic Materials—Part 1: Stainless Steel
ISO 8319-–1 Orthopaedic Instruments—Drive Connections —Part 1: Keys for Use with Screws with Hexagon Socket Heads
ISO 8319-–2 Orthopaedic Instruments—Drive Connections —Part 2: Screwdrivers for Single Slot Head Screws, screws with
Cruciate Slot, and Cross-Recessed Head Screws
ISO 9268 Implants for Surgery—Metal Bone Screws with Conical Under-Surface of Head—Dimensions
3. Classification
3.1 This specificati
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.