ASTM F2459-05
(Test Method)Standard Test Method for Extracting Residue from Metallic Medical Components and Quantifying via Gravimetric Analysis
Standard Test Method for Extracting Residue from Metallic Medical Components and Quantifying via Gravimetric Analysis
SIGNIFICANCE AND USE
This test method is suitable for determination of the extractable residue in metallic medical components. Extractable residue includes aqueous and non-aqueous residue, as well as non-soluble residue.
This test method recommends the use of a sonication technique to extract residue from the medical component. Other techniques, such as solvent reflux extraction, could be used but have been shown to be less efficient in some tests, as discussed in X1.2.
This test method is not applicable for evaluating the extractable residue for the reuse of a single-use component (SUD).
SCOPE
1.1 This test method covers the quantitative assessment of the amount of residue obtained from metallic medical components when extracted with aqueous or organic solvents.
1.2 This test method does not advocate an acceptable level of cleanliness. It identifies one technique to quantify extractable residue on metallic medical components. In addition, it is recognized that this test method may not be the only method to determine and quantify extractables.
1.3 Although these methods may give the investigator a means to compare the relative levels of component cleanliness, it is recognized that some forms of component residue may not be accounted for by these methods.
1.4 The applicability of these general gravimetric methods have been demonstrated by many literature reports; however, the specific suitability for applications to all-metal medical components will be validated by an Interlaboratory Study (ILS) conducted according to Practice E 691.
1.5 This test method is not intended to evaluate the residue level in medical components that have been cleaned for reuse. This test method is also not intended to extract residue for use in biocompatibility testing.
1.6 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.7 This standard may involve hazardous or environmentally-restricted materials, operations, and equipment. This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2459 – 05
Standard Test Method for
Extracting Residue from Metallic Medical Components and
Quantifying via Gravimetric Analysis
This standard is issued under the fixed designation F2459; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method covers the quantitative assessment of 2.1 ASTM Standards:
the amount of residue obtained from metallic medical compo- E691 Practice for Conducting an Interlaboratory Study to
nents when extracted with aqueous or organic solvents. Determine the Precision of a Test Method
1.2 This test method does not advocate an acceptable level G121 Practice for Preparation of Contaminated Test Cou-
of cleanliness. It identifies one technique to quantify extract- pons for the Evaluation of Cleaning Agents
able residue on metallic medical components. In addition, it is G131 Practice for Cleaning of Materials and Components
recognized that this test method may not be the only method to by Ultrasonic Techniques
determine and quantify extractables. G136 Practice for Determination of Soluble Residual Con-
1.3 Although these methods may give the investigator a taminants in Materials by Ultrasonic Extraction
means to compare the relative levels of component cleanliness,
3. Terminology
it is recognized that some forms of component residue may not
3.1 Definitions:
be accounted for by these methods.
1.4 The applicability of these general gravimetric methods 3.1.1 ionic compounds/water soluble residue—residue that
is soluble in water, including surfactants and salts.
have been demonstrated by many literature reports; however,
the specific suitability for applications to all-metal medical 3.1.2 non-soluble debris—residue including metals, organic
solids, inorganic solids, and ceramics.
componentswillbevalidatedbyanInterlaboratoryStudy(ILS)
conducted according to Practice E691. 3.1.3 non-water soluble residue—residue soluble in sol-
vents other than water. Inclusive in this are oils, greases,
1.5 This test method is not intended to evaluate the residue
hydrocarbons, and low molecular weight polymers. Typical
level in medical components that have been cleaned for reuse.
This test method is also not intended to extract residue for use solvents used to dissolve these residues include chlorinated or
fluorinated solvents, or low molecular weight hydrocarbons.
in biocompatibility testing.
1.6 The values stated in SI units are to be regarded as the 3.1.4 reflux system—an apparatus containing an extraction
vessel and a solvent return system. It is designed to allow
standard. The values given in parentheses are for information
only. boiling of the solvent in the extraction vessel and to return any
vaporized solvent to the extraction vessel.
1.7 This standard may involve hazardous or
environmentally-restricted materials, operations, and equip- 3.1.5 reuse—the repeated or multiple use of any medical
component (whether labeled SUD or reusable) with reprocess-
ment. This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the ing (cleaning, disinfection, or sterilization, or combination
thereof) between patient uses.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- 3.1.6 single use component (SUD)—a disposable compo-
nent; intended to be used on one patient during a single
bility of regulatory limitations prior to use.
procedure.
3.1.7 surface area—the projected surface area of a part.
This area does not include the internal porosity of parts with
This test method is under the jurisdiction ofASTM Committee F04 on Medical
cancellous, porous, or wire structure.
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.15 on Material Test Methods.
Current edition approved Aug. 1, 2005. Published August 2005. DOI: 10.1520/
F2459-05. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
For extraction of samples intended for the biological evaluation of devices or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
materials, refer to ISO 10993-12 Biological Evaluation—Sample Preparation and Standards volume information, refer to the standard’s Document Summary page on
Reference Materials. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2459 – 05
3.2 Symbols: 5. Significance and Use
5.1 This test method is suitable for determination of the
extractable residue in metallic medical components. Extract-
m = weight of extraction vessel and component before
ableresidueincludesaqueousandnon-aqueousresidue,aswell
extraction
as non-soluble residue.
m = weight of extraction vessel, component, and solvent
after extraction
5.2 This test method recommends the use of a sonication
m = mass of clean beaker used to hold removed aliquot of
3 technique to extract residue from the medical component.
extracted solution
Other techniques, such as solvent reflux extraction, could be
m = mass of beaker and aliquot of solution before drying
used but have been shown to be less efficient in some tests, as
m = mass of beaker and residue after evaporating solvent
discussed in X1.2.
m = mass of new filter
5.3 This test method is not applicable for evaluating the
m = mass of filter following filtration and drying
extractable residue for the reuse of a single-use component
m = mass of residue in removed aliquot
a
(SUD).
c = concentration of residue in solution
r
c = concentration of residue in blank solutions
b
m = mass of soluble residue in the overall extract, cor- 6. Apparatus
r
rected for the blank runs
6.1 Ultrasonic Bath, for extraction. The bath must be large
m = weight of insoluble debris
i
enough to hold an extraction beaker containing the medical
m = mass of soluble and insoluble residue
t
component. This apparatus is used with the technique de-
E = extraction efficiency
scribed in 11.5. Alternatively, an ultrasonic probe can be used
4. Summary of Test Method with a bath.
4.1 This test method describes the extraction and quantita- 6.2 Solvent Reflux Extraction Assembly, shown in Fig. 1.
Thisassemblyiscomposedofavessellargeenoughtoholdthe
tive analysis procedures used to detect and quantify extractable
residue from metallic medical components. The residues are medical component, and a water-cooled refluxing column. A
grouped into three categories: (1) water-soluble extractables; heating manifold or hotplate stirrer capable of reaching the
(2) non-water soluble extractables; and (3) non-soluble debris. boiling point of the solvent is also included. This apparatus is
FIG. 1 Sample Solvent Reflux Extractor Assembly
F2459 – 05
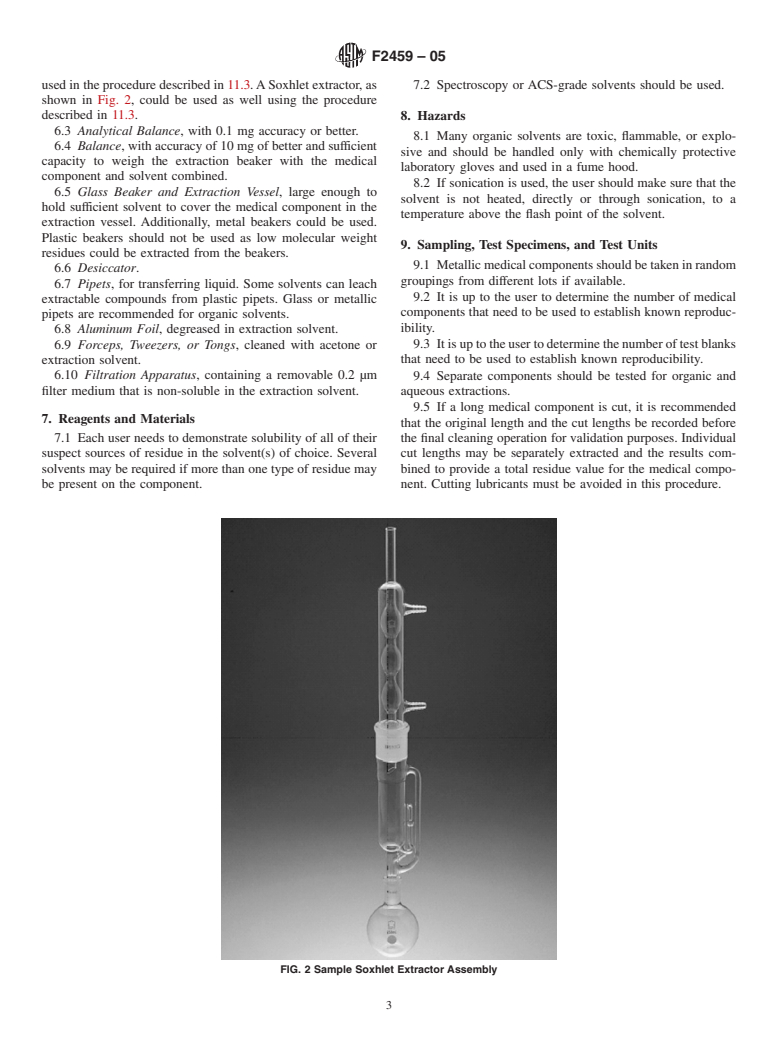
used in the procedure described in 11.3.ASoxhlet extractor, as 7.2 Spectroscopy or ACS-grade solvents should be used.
shown in Fig. 2, could be used as well using the procedure
described in 11.3.
8. Hazards
6.3 Analytical Balance, with 0.1 mg accuracy or better.
8.1 Many organic solvents are toxic, flammable, or explo-
6.4 Balance, with accuracy of 10 mg of better and sufficient
sive and should be handled only with chemically protective
capacity to weigh the extraction beaker with the medical
laboratory gloves and used in a fume hood.
component and solvent combined.
8.2 If sonication is used, the user should make sure that the
6.5 Glass Beaker and Extraction Vessel, large enough to
solvent is not heated, directly or through sonication, to a
hold sufficient solvent to cover the medical component in the
temperature above the flash point of the solvent.
extraction vessel. Additionally, metal beakers could be used.
Plastic beakers should not be used as low molecular weight
9. Sampling, Test Specimens, and Test Units
residues could be extracted from the beakers.
9.1 Metallicmedicalcomponentsshouldbetakeninrandom
6.6 Desiccator.
groupings from different lots if available.
6.7 Pipets, for transferring liquid. Some solvents can leach
9.2 It is up to the user to determine the number of medical
extractable compounds from plastic pipets. Glass or metallic
components that need to be used to establish known reproduc-
pipets are recommended for organic solvents.
ibility.
6.8 Aluminum Foil, degreased in extraction solvent.
6.9 Forceps, Tweezers, or Tongs, cleaned with acetone or 9.3 Itisuptotheusertodeterminethenumberoftestblanks
that need to be used to establish known reproducibility.
extraction solvent.
6.10 Filtration Apparatus, containing a removable 0.2 µm
9.4 Separate components should be tested for organic and
filter medium that is non-soluble in the extraction solvent. aqueous extractions.
9.5 If a long medical component is cut, it is recommended
7. Reagents and Materials
that the original length and the cut lengths be recorded before
7.1 Each user needs to demonstrate solubility of all of their the final cleaning operation for validation purposes. Individual
suspect sources of residue in the solvent(s) of choice. Several cut lengths may be separately extracted and the results com-
solvents may be required if more than one type of residue may bined to provide a total residue value for the medical compo-
be present on the component. nent. Cutting lubricants must be avoided in this procedure.
FIG. 2 Sample Soxhlet Extractor Assembly
F2459 – 05
10. Limits of Detection and Recovery Efficiency 11.3.14 Weigh an aliquot beaker large enough to hold an
aliquot of the extraction vessel along with a clean piece of foil
10.1 Standardized test coupons can be prepared according
and record the weight as m . The beaker should be weighed to
to Practice G121. Limits of detection with the two techniques 3
a resolution of at least 0.1 mg.
can be assessed by placing known amounts of residues on the
11.3.15 Allow the insoluble debris to settle in the extraction
test coupons, and performing the extraction and analyses
vessel for 1 h. Withdraw an aliquot of the extracted solution
described in Section 11.The recovery efficiency of the selected
that comprises at least 90 % of the total extracted solution and
extraction technique should be determined by doping pre-
place in the aliquot beaker as described in 11.3.14, being
cleaned medical components with known amounts of the target
carefulnottowithdrawanyinsolubledebrisfromthebottomof
residue, then extracting and quantifying the target residue. The
the extraction vessel. Weigh the solution with beaker and foil
extraction efficiency E is the ratio of the amount of recovered
and record as m .
residue to the doped amount of residue.
10.2 The user should adjust the extraction parameters in 11.3.15.1 Allow the solvent to completely evaporate in a
11.3.11 or 11.5.8, or select the appropriate solvent, or both, in
fume hood at room temperature. See X1.1.3 for more details.
order to achieve an extraction efficiency of E>75%.
11.3.15.2 Place the beaker, with residue, in a dessicator for
a minimum of 2 h.
11. Procedure
11.3.15.3 Weigh the beaker and foil again and record as m .
11.1 If more than one specimen is to be extracted collec-
11.3.15.4 If the volume of the aliquot beaker is smaller than
tively, record the number of specimens.
the aliquot, multiple aliquots can be removed from the extrac-
11.2 If multiple specimens are to be extracted collectively,
tionvessel,weighingeachaliquot,evaporatingthesolvent,and
they must be of the same type and size.
collecting the next aliquot. The solution weight m is the sum
11.3 Reflux Extraction:
of the aliquot weights plus the foil weight. The final beaker
11.3.1 Equipmentmayneedtobecleanedwithnitricacidor
weight m should be recorded as described in 11.3.15.3.
other appropriate means prior to solvent cleaning.
11.4 Blank Run:
11.3.2 Clean the extraction equipment by rinsing at least
11.4.1 Conduct test blank(s) using the same amount of
three times with spectroscopy-grade hexane or another suitable
solvent and rinses, but no component, for the complete
solvent. The extraction solvent may be used.
extractionandanalysisprocedure.Recordallweightsasabove.
11.3.3 Air dry all beakers and glassware at room tempera-
11.5 Sonication Extraction:
ture in a fume hood and store in a dessicator prior to use.
11.5.1 Backgroundinformationonsonicationextractioncan
11.3.4 Assemble the extraction apparatus as shown in Fig.
be found in Practices G131 and G136.
1.
11.5.2 Glassware may need to be cleaned with nitric acid or
11.3.5 Do not use any type of joint grease on the extraction
other appropriate means prior to solvent cleaning.
assembly. It can dissolve in the solvent and contaminate the
solution.Polytetrafluoroethylene(PTFE)sleevesortapecanbe 11.5.3 Clean the glassware by rinsing at least three times
used to seal the joints if necessary. with spectroscopy-grade hexane or another suitable solvent.
11.3.6 Place the sample component in the extractor vessel The extraction solvent may be used.
and add a magnetic stirring bar or PTFE boiling stones to
11.5.4 Air dry all beakers and glassware at room tempera-
reducethepotentialforboilingretardationinthesystemduring
ture in a fume hood and store in a dessicator prior to use.
reflux. The stir bar or boiling stones, or both, should be
11.5.5 Place the medical component in a beaker, cover with
carefully cleaned in a suitable solvent prior to use.
clean foil, and weigh. Record the weight as m .
11.3.7 Weigh the extractor vessel with the component on a
11.5.6 Add enough solvent to completely cover the compo-
balance and record the weight m .
nent.
11.3.8 Charge the flask with enough solvent to completely
11.5.7 Cover the beaker with the clean aluminum foil, then
cover the component(s) and assemble the reflux system.
place in a sonicator bath.The aluminum foil should not contact
11.3.9 Start flow of cooling water through the condenser.
the water in the sonicator bath.
11.3.10 Adjust the hotplate stirrer or heating manifold to
11.5.8 Startthesonicatorbath,andextractthecomponent(s)
maintain the solvent at a brisk boil with moderate constant
for a time period and temperature determined by the user
stirring.
pending internal validation of their extraction efficiency on the
11.3.11 Extract the component(s) for 4 h or for approxi-
targetresidues.Theextractiontemperatureshouldbebelowthe
mately 10 cycles if using a Soxhlet extractor. The extraction
boiling point of the solvent. More details on sonication times
time or number of
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.