ASTM F2952-22
(Guide)Standard Guide for Determining the Mean Darcy Permeability Coefficient for a Porous Tissue Scaffold
Standard Guide for Determining the Mean Darcy Permeability Coefficient for a Porous Tissue Scaffold
SIGNIFICANCE AND USE
4.1 This document describes the basic principles that need to be followed to obtain a mean value of the Darcy permeability coefficient for structures that consist of a series of interconnected voids or pores. The coefficient is a measure of the permeability of the structure to fluid flowing through it that is driven by a pressure gradient created across it.
4.2 The technique is not sensitive to the presence of closed or blind-end pores (Fig. 1).
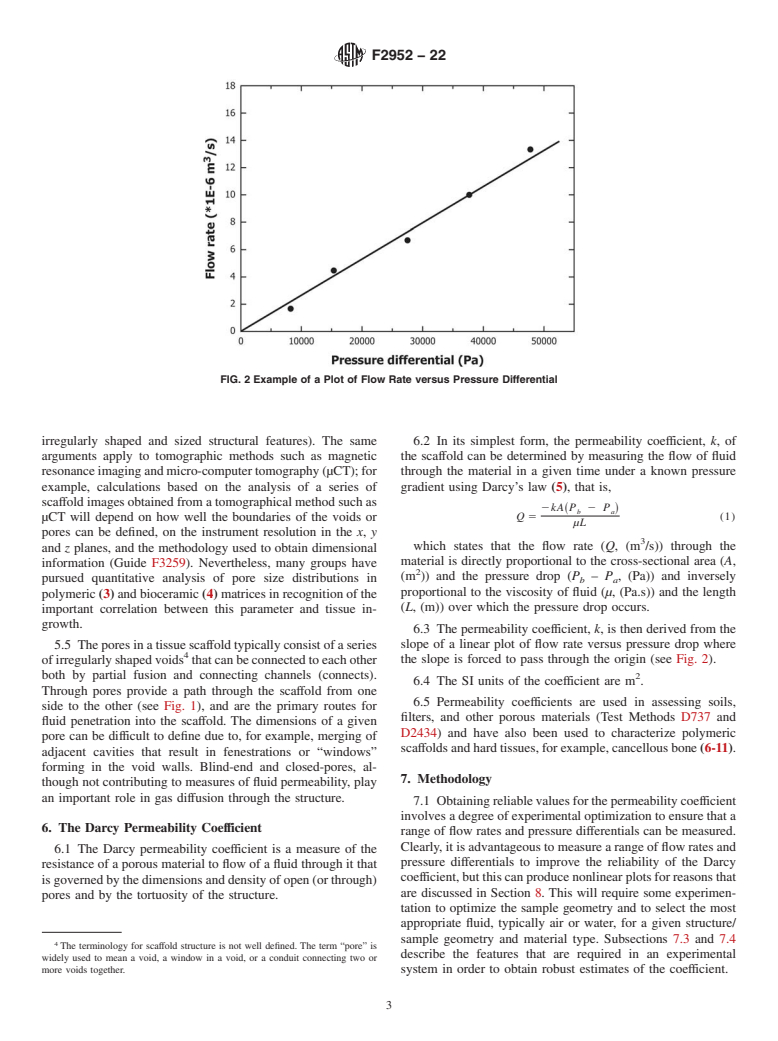
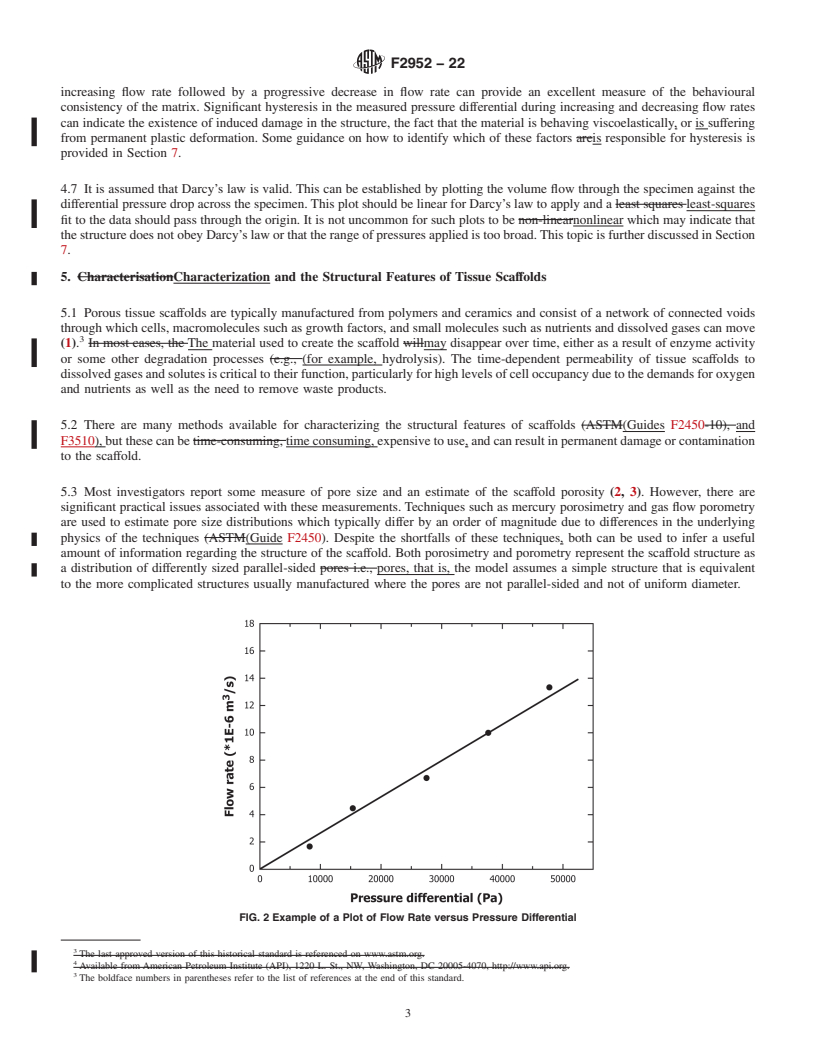
FIG. 1 Schematic of the Different Pores Types Found in Tissue Scaffolds. Fluid Flow Through the Structure is via the Open Pores
4.3 Values of the permeability coefficient can be used to compare the consistency of manufactured samples or to determine what the effect of changing one or more manufacturing settings has on permeability. They can also be used to assess the homogeneity and anisotropy of tissue scaffolds. Variability in the permeability coefficient can be also be indicative of:
4.3.1 Internal damage within the sample, for example, cracking or permanent deformation.
4.3.2 The presence of large voids, including trapped air bubbles, within the structure.
4.3.3 Surface effects such as a skin formed during manufacture.
4.3.4 Variable sample geometry.
4.4 This test method is based on the assumption that the flow rate through a given sample subjected to an applied pressure gradient is constant with time.
Note 1: If a steady-state flow condition isn’t reached, then this could be due to structural damage (that is, crack formation or the porous structure deformed as a result of the force being placed upon it by the fluid flowing through it). Sample deformation in the form of stretching (bowing) can also occur for less resilient structures as a result of high fluid flow rates. This topic is discussed in more detail in Section 7.
4.5 Care should be taken to ensure that hydrophobic materials are fully wetted out when using water or other aqueous-based liquids as permeants.
4.6 Conventionally, the p...
SCOPE
1.1 This guide describes test methods suitable for determining the mean Darcy permeability coefficient for a porous tissue scaffold, which is a measure of the rate at which a fluid, typically air or water, flows through it in response to an applied pressure gradient. This information can be used to optimize the structure of tissue scaffolds, to develop a consistent manufacturing process, and for quality assurance purposes.
1.2 The method is generally nondestructive and non-contaminating.
1.3 The method is not suitable for structures that are easily deformed or damaged. Some experimentation is usually required to assess the suitability of permeability testing for a particular material/structure and to optimize the experimental conditions.
1.4 Measures of permeability should not be considered as definitive metrics of the structure of porous tissue scaffolds and should complement measures obtained by other investigative techniques, for example, scanning electron microscopy, gas flow porometry, and micro-computer X-ray tomography (Guides F2450, F2603, and F3259).
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2952 − 22
Standard Guide for
Determining the Mean Darcy Permeability Coefficient for a
1
Porous Tissue Scaffold
This standard is issued under the fixed designation F2952; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
2.1 ASTM Standards:
1.1 This guide describes test methods suitable for determin-
D737 Test Method for Air Permeability of Textile Fabrics
ing the mean Darcy permeability coefficient for a porous tissue
D2434 Test Methods for Measurement of Hydraulic Con-
scaffold, which is a measure of the rate at which a fluid,
ductivity of Coarse-Grained Soils
typicallyairorwater,flowsthroughitinresponsetoanapplied
F2450 Guide for Assessing Microstructure of Polymeric
pressure gradient.This information can be used to optimize the
Scaffolds for Use in Tissue-Engineered Medical Products
structure of tissue scaffolds, to develop a consistent manufac-
F2603 Guide for Interpreting Images of Polymeric Tissue
turing process, and for quality assurance purposes.
Scaffolds
1.2 The method is generally nondestructive and non- F3259 Guide for Micro-computed Tomography of Tissue
contaminating. Engineered Scaffolds
F3510 Guide for Characterizing Fiber-Based Constructs for
1.3 The method is not suitable for structures that are easily
Tissue-Engineered Medical Products
deformed or damaged. Some experimentation is usually re-
quired to assess the suitability of permeability testing for a
3. Terminology
particular material/structure and to optimize the experimental
3.1 Definitions of Terms Specific to This Standard:
conditions.
3.1.1 tortuosity, n—the ratio of the actual path length
through connected pores to the Euclidean distance (shortest
1.4 Measures of permeability should not be considered as
linear distance).
definitivemetricsofthestructureofporoustissuescaffoldsand
should complement measures obtained by other investigative
4. Significance and Use
techniques, for example, scanning electron microscopy, gas
4.1 This document describes the basic principles that need
flow porometry, and micro-computer X-ray tomography
to be followed to obtain a mean value of the Darcy permeabil-
(Guides F2450, F2603, and F3259).
ity coefficient for structures that consist of a series of intercon-
1.5 This standard does not purport to address all of the
nected voids or pores. The coefficient is a measure of the
safety concerns, if any, associated with its use. It is the
permeability of the structure to fluid flowing through it that is
responsibility of the user of this standard to establish appro-
driven by a pressure gradient created across it.
priate safety, health, and environmental practices and deter-
4.2 The technique is not sensitive to the presence of closed
mine the applicability of regulatory limitations prior to use.
or blind-end pores (Fig. 1).
1.6 This international standard was developed in accor-
4.3 Values of the permeability coefficient can be used to
dance with internationally recognized principles on standard-
compare the consistency of manufactured samples or to deter-
ization established in the Decision on Principles for the
mine what the effect of changing one or more manufacturing
Development of International Standards, Guides and Recom-
settings has on permeability. They can also be used to assess
mendations issued by the World Trade Organization Technical
the homogeneity and anisotropy of tissue scaffolds. Variability
Barriers to Trade (TBT) Committee.
in the permeability coefficient can be also be indicative of:
4.3.1 Internal damage within the sample, for example,
cracking or permanent deformation.
1
This guide is under the jurisdiction of ASTM Committee F04 on Medical and
Surgical Materials and Devices and is the direct responsibility of Subcommittee
2
F04.42 on Biomaterials and Biomolecules for TEMPs. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved April 1, 2022. Published April 2022. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2014. Last previous edition approved in 2014 as F2952 – 14. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F2952-22. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2952 − 22
FIG. 1 Schematic of th
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2952 − 14 F2952 − 22
Standard Guide for
Determining the Mean Darcy Permeability Coefficient for a
1
Porous Tissue Scaffold
This standard is issued under the fixed designation F2952; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide describes test methods suitable for determining the mean Darcy permeability coefficient for a porous tissue scaffold,
which is a measure of the rate at which a fluid, typically air or water, flows through it in response to an applied pressure gradient.
This information can be used to optimize the structure of tissue scaffolds, to develop a consistent manufacturing process, and for
quality assurance purposes.
1.2 The method is generally non-destructivenondestructive and non-contaminating.
1.3 The method is not suitable for structures that are easily deformed or damaged. Some experimentation is usually required to
assess the suitability of permeability testing for a particular material/structure and to optimize the experimental conditions.
1.4 Measures of permeability should not be considered as definitive metrics of the structure of porous tissue scaffolds and should
complement measures obtained by other investigative techniques e.g., techniques, for example, scanning electron microscopy, gas
flow porometry, and micro-computer x-rayX-ray tomography (ASTM(Guides F2450, F2603, and F3259).
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
D4525D737 Test Method for Air Permeability of Rocks by Flowing AirTextile Fabrics (Withdrawn 2022)
D2434 Test Methods for Measurement of Hydraulic Conductivity of Coarse-Grained Soils
F2450 Guide for Assessing Microstructure of Polymeric Scaffolds for Use in Tissue-Engineered Medical Products
F2603 Guide for Interpreting Images of Polymeric Tissue Scaffolds
F3259 Guide for Micro-computed Tomography of Tissue Engineered Scaffolds
F3510 Guide for Characterizing Fiber-Based Constructs for Tissue-Engineered Medical Products
1
This test method guide is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.42 on Biomaterials and Biomolecules for TEMPs.
Current edition approved March 1, 2014April 1, 2022. Published April 2014April 2022. Originally approved in 2014. Last previous edition approved in 2014 as
F2952 – 14. DOI: 10.1520/F2952-14.10.1520/F2952-22.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2952 − 22
4
2.2 American Petroleum Institute (API) Document:
RP-27 Recommended Practice for Determining Permeability of Porous Media
3. Terminology
3.1 Definitions:Definitions of Terms Specific to This Standard:
3.1.1 tortuosity, n—the ratio of the actual path length through connected pores to the Euclidean distance (shortest linear distance).
4. Significance and Use
4.1 This document describes the basic principles that need to be followed to obtain a mean value of the Darcy permeability
coefficient for structures that consist of a series of interconnected voids or pores. The coefficient is a measure of the permeability
of the structure to fluid flowing through it that is driven by a pressure gradient created across it.
4.2 The technique is not sensitive to the presence of closed or blind-end pores (Fig. 1).
4.3 Values of the permeability coefficient can be used to compa
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.