ASTM F1113-87(2017)
(Test Method)Standard Test Method for Electrochemical Measurement of Diffusible Hydrogen in Steels (Barnacle Electrode)
Standard Test Method for Electrochemical Measurement of Diffusible Hydrogen in Steels (Barnacle Electrode)
SIGNIFICANCE AND USE
4.1 The critical level of hydrogen in steels is that hydrogen which can build up to high concentrations at points of high triaxial stress causing embrittlement of the steel which can lead to catastrophic damage. This hydrogen can enter by various means, such as during pickling and electroplating. Means of reducing this hydrogen during processing are given in Specification B766 and Practices B183 and B242. It is still necessary, however, to know how effective these methods are. Though the ultimate reason for measuring this hydrogen is to relate it to embrittlement, this is not within the scope of this test method. As susceptibility to hydrogen embrittlement is a function of alloy type, heat treatment, intended use,and so forth, the tolerance for hydrogen must be determined by the user according to Method F519.
4.2 Though the actual hydrogen concentration is not determined in this test method, the current densities have been shown to be useful as an indication of relative hydrogen concentrations (1-3),3 and therefore the degree of hydrogen embrittlement (1,2). Thus, measurements can be compared to one another (see 4.1 and 7.1).
4.3 This test method is applicable as a quality control tool for processing (such as to monitor plating and baking) or to measure hydrogen uptake caused by corrosion.
4.4 This test method is nondestructive; however, if there is a coating, it must be removed by a method which has been demonstrated to neither damage the steel nor introduce hydrogen to make the measurement.
4.5 This test method is also applicable to situations producing continuous hydrogen permeation, such as high pressure hydrogen cylinders or corrosion processes. The results, however, would require a different treatment and interpretation (4).
4.6 This test method is also applicable to small parts, such as fasteners. The technique, procedure, and interpretation would, however, have to be altered.
4.7 Use of this test method on austenitic stainless steels and o...
SCOPE
1.1 This test method covers the procedure for measuring diffusible hydrogen in steels by an electrochemical method.
1.2 This test method is limited to carbon or alloy steels, excluding austenitic stainless steels.
1.3 This test method is limited to flat specimens to which the cell can be attached (see 4.6 and 4.8).
1.4 This test method describes testing on bare or plated steel after the plate has been removed (see 4.4).
1.5 This test method is limited to measurements at room temperature, 20 to 25°C (68 to 77°F).
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F1113 − 87 (Reapproved 2017)
Standard Test Method for
Electrochemical Measurement of Diffusible Hydrogen in
Steels (Barnacle Electrode)
This standard is issued under the fixed designation F1113; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D1193Specification for Reagent Water
F519Test Method for Mechanical Hydrogen Embrittlement
1.1 This test method covers the procedure for measuring
Evaluation of Plating/Coating Processes and Service En-
diffusible hydrogen in steels by an electrochemical method.
vironments
1.2 This test method is limited to carbon or alloy steels,
G3Practice for Conventions Applicable to Electrochemical
excluding austenitic stainless steels.
Measurements in Corrosion Testing
1.3 This test method is limited to flat specimens to which
3. Summary of Test Method
the cell can be attached (see 4.6 and 4.8).
3.1 A hydrogen-containing part is made the anode in an
1.4 Thistestmethoddescribestestingonbareorplatedsteel
electrochemical cell. The diffusible (atomic) hydrogen, which
after the plate has been removed (see 4.4).
comes to the metal-electrolyte interface, is oxidized to protons
+ + −
1.5 This test method is limited to measurements at room
(H );H combineswithhydroxylions(OH )intheelectrolyte
temperature, 20 to 25°C (68 to 77°F).
toformwater.Theoxidationcurrentismeasuredandrelatedto
1.6 This standard does not purport to address all of the
the hydrogen concentration.
safety concerns, if any, associated with its use. It is the
4. Significance and Use
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
4.1 The critical level of hydrogen in steels is that hydrogen
bility of regulatory limitations prior to use.
which can build up to high concentrations at points of high
1.7 This international standard was developed in accor-
triaxialstresscausingembrittlementofthesteelwhichcanlead
dance with internationally recognized principles on standard-
to catastrophic damage. This hydrogen can enter by various
ization established in the Decision on Principles for the
means, such as during pickling and electroplating. Means of
Development of International Standards, Guides and Recom-
reducing this hydrogen during processing are given in Speci-
mendations issued by the World Trade Organization Technical
fication B766 and Practices B183 and B242. It is still
Barriers to Trade (TBT) Committee.
necessary, however, to know how effective these methods are.
Though the ultimate reason for measuring this hydrogen is to
2. Referenced Documents
relateittoembrittlement,thisisnotwithinthescopeofthistest
method. As susceptibility to hydrogen embrittlement is a
2.1 ASTM Standards:
function of alloy type, heat treatment, intended use,and so
B183Practice for Preparation of Low-Carbon Steel for
forth, the tolerance for hydrogen must be determined by the
Electroplating
user according to Method F519.
B242Guide for Preparation of High-Carbon Steel for Elec-
troplating
4.2 Though the actual hydrogen concentration is not deter-
B766Specification for Electrodeposited Coatings of Cad-
mined in this test method, the current densities have been
mium
shown to be useful as an indication of relative hydrogen
concentrations (1-3), and therefore the degree of hydrogen
embrittlement (1,2). Thus, measurements can be compared to
This test method is under the jurisdiction of ASTM Committee F07 on
one another (see 4.1 and 7.1).
Aerospace andAircraft and is the direct responsibility of Subcommittee F07.04 on
Hydrogen Embrittlement.
4.3 This test method is applicable as a quality control tool
CurrenteditionapprovedJune1,2017.PublishedJuly2017.Originallyapproved
for processing (such as to monitor plating and baking) or to
in 1987. Last previous edition approved in 2011 as F1113 – 87 (2011). DOI:
10.1520/F1113-87R17. measure hydrogen uptake caused by corrosion.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Boldfacenumbersinparenthesesrefertothelistofreferencesattheendofthis
the ASTM website. standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F1113 − 87 (2017)
4.4 This test method is nondestructive; however, if there is
a coating, it must be removed by a method which has been
demonstrated to neither damage the steel nor introduce hydro-
gen to make the measurement.
4.5 This test method is also applicable to situations produc-
ing continuous hydrogen permeation, such as high pressure
hydrogen cylinders or corrosion processes. The results,
however,wouldrequireadifferenttreatmentandinterpretation
(4).
4.6 This test method is also applicable to small parts, such
as fasteners. The technique, procedure, and interpretation
would, however, have to be altered.
4.7 Use of this test method on austenitic stainless steels and
other face centered cubic (FCC) alloys would require different
measurement times and interpretation of results because of
differing kinetics.
4.8 Thistestmethodcanbeusedonslightlycurvedsurfaces
as long as the gasket defines a reproducible area. The area
calculation must, however, be changed.
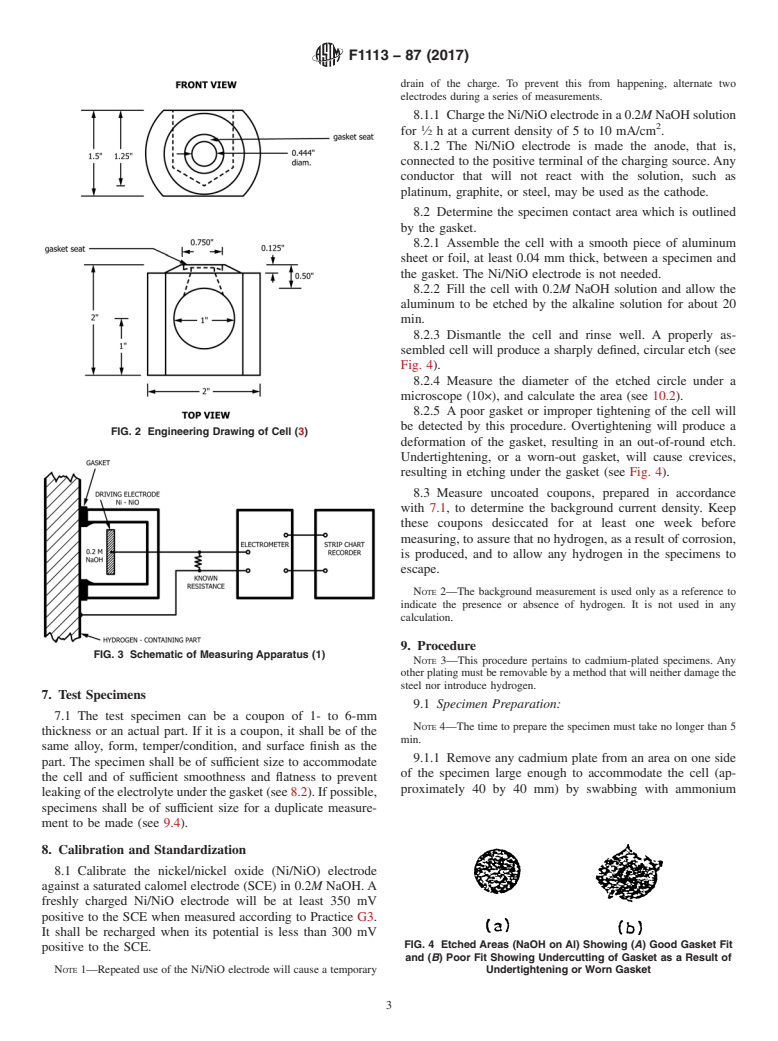
5. Apparatus
5.1 Cell—A photo and drawing of a typical cell, which has
been found to be acceptable for hydrogen measurements, are
shown in Figs. 1 and 2, respectively.
5.1.1 Thecellismadeofanonmetallicmaterialthatwillnot
react with or contaminate the solution. The side opening has a
recess to accommodate the silicone rubber gasket.
5.1.2 Gasket, silicone rubber, shall provide a reproducible
solution-contact area with the specimen, preferably 1.0 cm .
5.1.3 Cell Holder, a cradle-like C-clamp. Other clamping
devices can be used if necessary, such as for larger parts.
5.1.4 Cathode,anickel/nickeloxideelectrode.Itismadeby
FIG. 1 Photograph of Cell
removingthepositiveplatefromanickel/cadmiumbatteryand
attachinganickelwireorfoil.Theareaofthiscathodeshallbe
approximately five times that of the anode.
all reagents shall conform to the specifications of the Commit-
5.1.5 Anode—The anode is the specimen.
tee onAnalytical Reagents of theAmerican Chemical Society,
5.1.6 The cell is left open to the atmosphere. No purging is
where such specifications are available. Other grades may be
used.
used, provided it is first ascertained that the reagent is of
5.2 Current Measuring Device—The current can be mea-
sufficiently high purity to permit its use without lessening the
sured by any method that will not affect its value. A zero
accuracy of the determination.
resistance ammeter (5), a current follower (6), and the current
6.2 Purity of Water—Distilled or deionized water conform-
measuring system shown in Fig. 3 (1) have been found to be
ing to Specification D1193, Type IV, shall be used to prepare
acceptable. The following description refers to Fig. 3.
all solutions.
5.2.1 Standard Resistor, connected across the cell through a
6.3 Sodium Hydroxide Solution (0.2M)—Dissolve8gof
switch.
sodium hydroxide (NaOH) pellets in water and dilute to 1 L.
5.2.2 Electrometer, to determine the current by measuring
the voltage drop across the resistor. A 10-kΩ resistor with an
6.4 Ammonium Nitrate Solution (120 g/L)—Dissolve 120 g
electrometer having an input impedance of 10 Ω and a 1-mA
of ammonium nitrate (NH NO ) in water and dilute to 1 L.
4 3
output has been found to be satisfactory.
6.5 Methyl Alcohol (CH OH).
5.2.3 Strip Chart Recorder, to monitor the electrometer
6.6 Ethyl Alcohol (C H OH).
output. A recorder having an input resistance of 100 kΩ has
2 5
been found to be satisfactory.
5.2.4 Timer, accurate to within 10 s in a 30-min run.
Reagent Chemicals, American Chemical Society Specifications, American
6. Reagents Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, seeAnalar Standards for Laboratory
6.1 Purity of Reagents—Reagent grade chemicals shall be
Chemicals,BDHLtd.,Poole,Dorset,U.K.,andtheUnited States Pharmacopeia and
used in all tests. Unless otherwise indicated, it is intended that National Formulary,U.S.PharmacopeialConvention,Inc.(USPC),Rockville,MD.
F1113 − 87 (2017)
drain of the charge. To prevent this from happening, alternate two
electrodes during a series of measurements.
8.1.1 ChargetheNi/NiOelectrodeina0.2MNaOHsolution
for ⁄2 h at a current density of 5 to 10 mA/cm .
8.1.2 The Ni/NiO electrode is made the anode, that is,
connected to the positive terminal of the charging source.Any
conductor that will not react with the solution, such as
platinum, graphite, or steel, may be used as the cathode.
8.2 Determine the specimen contact area which is outlined
by the gasket.
8.2.1 Assemble the cell with a smooth piece of aluminum
sheet or foil, at least 0.04 mm thick, between a specimen and
the gasket. The Ni/NiO electrode is not needed.
8.2.2 Fill the cell with 0.2M NaOH solution and allow the
aluminum to be etched by the alkaline solution for about 20
min.
8.2.3 Dismantle the cell and rinse well. A properly as-
sembled cell will produce a sharply defined, circular etch (see
Fig. 4).
8.2.4 Measure the diameter of the etched circle under a
microscope (10×), and calculate the area (see 10.2).
8.2.5 A poor gasket or improper tightening of the cell will
be detected by this procedure. Overtightening will produce a
FIG. 2 Engineering Drawing of Cell (3)
deformation of the gasket, resulting in an out-of-round etch.
Undertightening, or a worn-out gasket, will cause crevices,
resulting in etching under the gasket (see Fig. 4).
8.3 Measure uncoated coupons, prepared in accordance
with 7.1, to determine the background current density. Keep
these coupons desiccated for at least one week before
measuring, to assure that no hydrogen, as a result of corrosion,
is produced, and to allow any hydrogen in the specimens to
escape.
NOTE 2—The background measurement is used only as a reference to
indicate the presence or absence of hydrogen. It is not used in any
calculation.
9. Procedure
FIG. 3 Schematic of Measuring Apparatus (1)
NOTE 3—This procedure pertains to cadmium-plated specimens. Any
other plating must be removable by a method that will neither damage the
steel nor introduce hydrogen.
7. Test Specimens
9.1 Specimen Preparation:
7.1 The test specimen can be a coupon of 1- to 6-mm
NOTE 4—The time to prepare the specimen must take no longer than 5
thickness or an actual part. If it is a coupon, it shall be of the
min.
same alloy, form, temper/condition, and surface finish as the
9.1.1 Remove any cadmium plate from an area on one side
part. The specimen shall be of sufficient size to accommodate
of the specimen large enough to accommodate the cell (ap-
the cell and of sufficient smoothness and flatness to prevent
proximately 40 by 40 mm) by swabbing with ammonium
leakingoftheelectrolyteunderthegasket(see8.2).Ifpossible,
specimens shall be of sufficient size for a duplicate measure-
ment to be made (see 9.4).
8. Calibration and Standardization
8.1 Calibrate the nickel/nickel oxide (Ni/NiO) electrode
against a saturated calomel electrode (SCE) in 0.2M NaOH.A
freshly charged Ni/NiO electrode will be at least 350 mV
positive to the SCE when measured according to Practice G3.
It shall be recharged when its potential is less than 300 mV
FIG. 4 Etched Areas (NaOH on Al) Showing (A) Good Gasket Fit
positive to the SCE.
and (B) Poor Fit Showing Undercutting of Gasket as a Result of
NOTE 1—Repeated use of the Ni/NiO electrode will cause a temporary Undertightening or Worn Gasket
F1113 − 87 (2017)
nitrate solution. Rinse with water and dry. Swabs made of
polyurethane foam or cotton have been found to be satisfac-
tory.
9.1.2 Abrade the surface lightly with an aluminum oxide-
impregnated nylon cleaning pad to remove surface contamina-
tion and to provide a reproducible surface finish. Wipe clean
using a tissue wet with methyl or ethyl alcohol.
9.2 Cell Assembly:
NOTE 5—The time to assemble the cell and start the measurement must
take no longer than 5 min. The total time from the start of 9.1.1 – 9.3.1
must take no longer than 10 min.
9.2.1 Clamp the Cell to the Specimen.
NOTE 6—The cell should be clamped only tight enough to prevent
leakage. Overtightening will cause deformation of the gasket. Proper
tightening can be determined by following the procedure in 8.2.
9.2.2 Clamp the Ni/NiO electrode in the center of the cell
cavity using the cell dimensions of Fig. 1. For other cell
designs, the distance between the electrodes shall be 25 mm.
9.2.3 Connect the resistor and switch between the Ni/NiO
electrode and the specimen.
9.2.4 Connecttheelectrometeracrosstheresistorsothatthe
Ni/NiO electrode will measure positive and the steel negative.
9.2.5 Connect the recorder to the electrometer output.
9.2.6 Fill the cell with 0.2M NaOH, making sure that the
Ni/NiO electrode and the specimen measurement area are
completely covered with solution.
9.3 Making the Measurement:
NOTE 7—The measurement must be started within 1 min of filling the
FIG. 5 Recorder Tracings Showing (A) Good Measurement, (B )
cell.
Acceptable Measurement, and (C ), Poor Measurement Which
9.3.1 Simultaneously turn on the cell switch and the timer.
Must Be Repeated
NOTE 8—The oxidation current decreases with time. During the
measurement, it will change by a few orders of magnitude. Therefore, for
10. Calculation
the first 5 min, set the recorder at an appropriate high setting to prevent
10.1 Calculate the current from the voltage drop across the
overload. The final readings will be in the microampere range.Adjust the
electrometer and recorder accordingly.
standardresistorusingOhm’slaw,I=E⁄R.Forexample,ifthe
full-scale voltage on the strip chart recorder is 10 mV and the
9.3.2 Record the current at the end of 30 min. This shall be
resistance is 10 kΩ, then the full-scale current is 1 µA.
referred to as the 30-min reading.
10.2 Calculate the contact area from the diameter of the
NOTE 9—The current measurement must always be made for the same
etched surface (see 8.2). The diameter, D, should be measured
length of time. In this test method, 30 min has been chosen. The reasons
in t
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.