ASTM G3-89(2010)
(Practice)Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing
Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing
SIGNIFICANCE AND USE
This practice provides guidance for reporting, displaying, and plotting electrochemical corrosion data and includes recommendations on signs and conventions. Use of this practice will result in the reporting of electrochemical corrosion data in a standard format, facilitating comparison between data developed at different laboratories or at different times. The recommendations outlined in this standard may be utilized when recording and reporting corrosion data obtained from electrochemical tests such as potentiostatic and potentiodynamic polarization, polarization resistance, electrochemical impedance and admittance measurements, galvanic corrosion, and open circuit potential measurements.
SCOPE
1.1 This practice covers conventions for reporting and displaying electrochemical corrosion data. Conventions for potential, current density, electrochemical impedance and admittance, as well as conventions for graphical presentation of such data are included.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard. See also 7.4.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G3 − 89(Reapproved 2010)
Standard Practice for
Conventions Applicable to Electrochemical Measurements
in Corrosion Testing
This standard is issued under the fixed designation G3; the number immediately following the designation indicates the year of original
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope impedance and admittance measurements, galvanic corrosion,
and open circuit potential measurements.
1.1 This practice covers conventions for reporting and
displaying electrochemical corrosion data. Conventions for
4. Sign Convention for Electrode Potential
potential, current density, electrochemical impedance and
4.1 The Stockholm sign invariant convention is recom-
admittance, as well as conventions for graphical presentation
mended for use in reporting the results of specimen potential
of such data are included.
measurements in corrosion testing. In this convention, the
1.2 The values stated in SI units are to be regarded as
positivedirectionofelectrodepotentialimpliesanincreasingly
standard. No other units of measurement are included in this
oxidizing condition at the electrode in question. The positive
standard. See also 7.4.
direction has also been denoted as the noble direction because
1.3 This standard does not purport to address all of the
thecorrosionpotentialsofmostnoblemetals,suchasgold,are
safety concerns, if any, associated with its use. It is the
more positive than the nonpassive base metals. On the other
responsibility of the user of this standard to establish appro-
hand,thenegativedirection,oftencalledtheactivedirection,is
priate safety and health practices and determine the applica-
associated with reduction and consequently the corrosion
bility of regulatory limitations prior to use.
potentials of active metals, such as magnesium. This conven-
tionwasadoptedunanimouslybythe1953InternationalUnion
2. Referenced Documents
of Pure and Applied Chemistry as the standard for electrode
2 3
2.1 ASTM Standards:
potential (1).
IEEE/ASTM SI 10Standard for Use of the International
4.2 In the context of a specimen electrode of unknown
System of Units (SI) (the Modern Metric System)
potential in an aqueous electrolyte, consider the circuit shown
in Fig. 1 with a reference electrode connected to the ground
3. Significance and Use
terminal of an electrometer. If the electrometer reads on scale
3.1 This practice provides guidance for reporting,
when the polarity switch is negative, the specimen electrode
displaying, and plotting electrochemical corrosion data and
potential is negative (relative to the reference electrode).
includes recommendations on signs and conventions. Use of
Conversely, if the electrometer reads on scale when polarity is
this practice will result in the reporting of electrochemical
positive, the specimen potential is positive. On the other hand,
corrosion data in a standard format, facilitating comparison
if the specimen electrode is connected to the ground terminal,
between data developed at different laboratories or at different
the potential will be positive if the meter is on scale when the
times. The recommendations outlined in this standard may be
polarity switch is negative, and vice versa.
utilized when recording and reporting corrosion data obtained
NOTE 1—In cases where the polarity of a measuring instrument is in
from electrochemical tests such as potentiostatic and potentio-
doubt, a simple verification test can be performed as follows: connect the
dynamic polarization, polarization resistance, electrochemical
measuring instrument to a dry cell with the lead previously on the
referenceelectrodetothenegativebatteryterminalandtheleadpreviously
1 on the specimen electrode to the positive battery terminal. Set the range
This practice is under the jurisdiction ofASTM Committee G01 on Corrosion
switchtoaccommodatethedrycellvoltage.Themeterdeflectionwillnow
ofMetalsandisthedirectresponsibilityofSubcommitteeG01.11onElectrochemi-
show the direction of positive potential.
cal Measurements in Corrosion Testing.
Also, the corrosion potential of magnesium or zinc should be negative
Current edition approved May 1, 2010. Published May 2010. Originally
ina1 N NaCl solution if measured against a saturated standard calomel
approved in 1968. Last previous edition approved in 2004 as G3–89(2004). DOI:
10.1520/G0003-89R10. electrode (SCE).
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The boldface numbers in parentheses refer to a list of references at the end of
the ASTM website. this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G3 − 89 (2010)
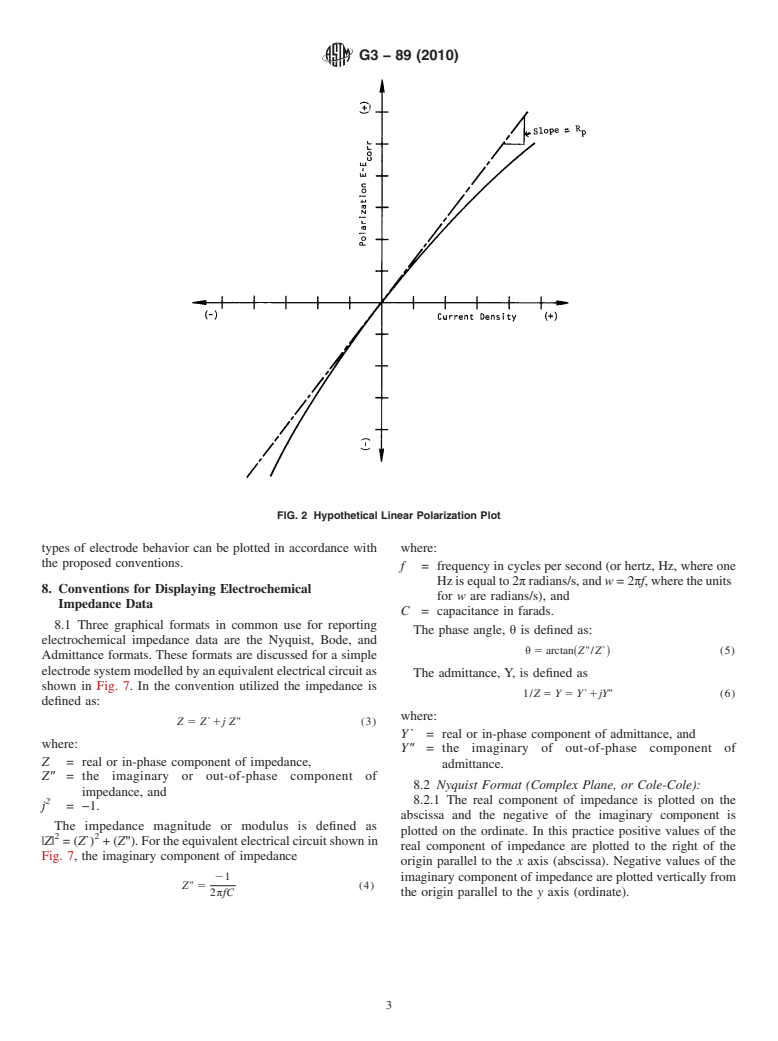
where the region in which the current density changes from
anodic to cathodic is important. Linear plots are also used for
the determination of the polarization resistance R , which is
p
defined as the slope of a potential-current density plot at the
corrosion potential E . The relationship between the polar-
corr
izationresistanceR andthecorrosioncurrentdensityi isas
p corr
follows (2, 3):
d~∆E! b b
a c
5 R 5 (1)
F G
p
di 2.303 b 1b i
~ !
∆E50 a c corr
where:
b = anodic Tafel slope,
a
b = cathodic Tafel slope, and
c
∆E = the difference E−E , where E is the specimen
corr
potential.
NOTE 1—The electrode potential of specimen is negative as shown.
Fig.2isaplotofpolarization, E−E ,versuscurrentdensity
corr
FIG. 1 Schematic Diagram of an Apparatus to Measure Electrode
i(solidline)fromwhichthepolarizationresistanceR hasbeen
Potential of a Specimen
p
determined as the slope of the curve at the corrosion potential
E .
corr
5. Sign Convention for Electrode Potential Temperature
7.3 Potential Reference Points—In plots where electrode
Coefficients
potentials are displayed, some indication of the conversion of
5.1 There are two types of temperature coefficients of
the values displayed to both the standard hydrogen electrode
electrode potential: isothermal temperature coefficients and the
scale (SHE) and the saturated calomel electrode scale (SCE) is
thermal coefficients. The sign convention recommended for
recommended if they are known. For example, when electrode
both types of temperature coefficients is that the temperature
potentialisplottedastheordinate,thentheSCEscalecouldbe
coefficient is positive when an increase in temperature pro-
shown at the extreme left of the plot and the SHE scale shown
duces an increase (that is, it becomes more positive) in the
at the extreme right. An alternative, in cases where the
electrode potential. Likewise, the second temperature coeffi-
reference electrode was not either SCE or SHE, would be to
cient is positive when an increase in temperature produces an
show on the potential axis the potentials of these electrodes
increase (that is, it becomes more positive) in the first tem-
against the reference used. In cases where these points are not
perature coefficient.
shown on the plot, an algebraic conversion could be indicated.
For example, in the case of a silver-silver chloride reference
6. Sign Convention for Current and Current Density
electrode(1MKCl),theconversioncouldbeshowninthetitle
6.1 The sign convention in which anodic currents and
box as:
current densities are considered positive and cathodic currents
SCE 5 E 2 0.006V (2)
and current densities are negative is recommended. When the
potential is plotted against the logarithm of the current density,
SHE 5 E10.235V
only the absolute values of the current density can be plotted.
where E represents electrode potential measured against the
In such plots, the values which are cathodic should be clearly
silver-silver chloride standard (1 M KCl).
differentiated from the anodic values if both are present.
NOTE2—Atableofpotentialsforvariouscommonreferenceelectrodes
is presented in Appendix X2.
7. Conventions for Displaying Polarization Data
7.4 Units—Therecommendedunitofpotentialisthevolt.In
7.1 Sign Conventions—The standard mathematical practice
cases where only small potential ranges are covered, millivolts
for plotting graphs is recommended for displaying electro-
or microvolts may be used.The SI units for current density are
chemical corrosion data. In this practice, positive values are
ampere per square metre or milliampere per square centimetre
plotted above the origin on the ordinate axis and to the right of
(IEEE/ASTM SI 10). Still in use are units expressed in
the origin on the abscissa axis. In logarithmic plots, the
amperes per square centimetre, and microamperes per square
abscissa value increases from left to right and the ordinate
centimetre.
value increases from bottom to top.
7.5 Sample Polarization Curves—Sample polarization plots
7.2 Current Density-Potential Plots—Auniform convention
employing these recommended practices are shown in Figs.
is recommended for plotting current density-potential data,
2-6. Fig. 3 and Fig. 4 are hypothetical curves showing active
namely, plot current density along the abscissa and potential
andactive-passiveanodebehavior,respectively.Fig.5andFig.
along the ordinate. In current density potential plots, the
currentdensitymaybeplottedonlinearorlogarithmicaxes.In 6areactualpolarizationdataforType430stainlesssteel(UNS
43000) (4)andtwoaluminumsamples (5).Fig.3andFig.4are
general, logarithmic plots are better suited to incorporation of
widerangesofcurrentdensitydataandfordemonstratingTafel exhibitedtoillustrategraphicallythelocationofvariouspoints
relationships. Linear plots are recommended for studies in used in discussion of electrochemical methods of corrosion
whichthecurrentdensityorpotentialrangeissmall,orincases testing.ThepurposeofFig.5andFig.6istoshowhowvarious
G3 − 89 (2010)
FIG. 2 Hypothetical Linear Polarization Plot
types of electrode behavior can be plotted in accordance with where:
the proposed conventions.
f = frequency in cycles per second (or hertz, Hz, where one
Hzisequalto2πradians/s,andw=2πf,wheretheunits
8. Conventions for Displaying Electrochemical
for w are radians/s), and
Impedance Data
C = capacitance in farads.
8.1 Three graphical formats in common use for reporting
The phase angle, θ is defined as:
electrochemical impedance data are the Nyquist, Bode, and
θ 5 arctan~Z"/Z`! (5)
Admittance formats. These formats are discussed for a simple
electrodesystemmodelledbyanequivalentelectricalcircuitas
The admittance, Y, is defined as
shown in Fig. 7. In the convention utilized the impedance is
1/Z 5 Y 5 Y`1jY" (6)
defined as:
where:
Z 5 Z`1jZ" (3)
Y` = real or in-phase component of admittance, and
where:
Y" = the imaginary of out-of-phase component of
Z = real or in-phase component of impedance,
admittance.
Z" = the imaginary or out-of-phase component of
8.2 Nyquist Format (Complex Plane, or Cole-Cole):
impedance, and
2 8.2.1 The real component of impedance is plotted on the
j = −1.
abscissa and the negative of the imaginary component is
The impedance magnitude or modulus is defined as
plotted on the ordinate. In this practice positive values of the
2 2
|Z| =(Z`) +(Z").Fortheequivalentelectricalcircuitshownin
real component of impedance are plotted to the right of the
Fig. 7, the imaginary component of impedance
origin parallel to the x axis (abscissa). Negative values of the
imaginary component of impedance are plotted vertically from
Z" 5 (4)
2πfC the origin parallel to the y axis (ordinate).
G3 − 89 (2010)
FIG. 3 Hypothetical Cathodic and Anodic Polarization Diagram
FIG. 4 Hypothetical Cathodic and Anodic Polarization Plots for a Passive Anode
G3 − 89 (2010)
FIG. 5 Typical Potentiostatic Anodic Polarization Plot for Type 430 Stainless Steel in 1.0N H SO
2 4
FIG. 6 Typical Polarization Plots for Aluminum Materials in 0.2N NaCl Solution
8.2.2 Fig. 8 shows a Nyquist plot for the equivalent circuit frequencypointscorrespondtotheincreasingmagnitudeofthe
of Fig. 7. The frequency dependence of the data is not shown impedance components.
explicitly on this type of plot. However, the frequency corre- 8.2.3 Recommended units for both axes are ohm·cm . The
sponding to selected data points may be directly annotated on units ohm·cm are obtained by multiplying the measured
the Nyquist plot. The magnitude of the appropriate impedance resistance or impedance by the exposed specimen area. For a
components increases when moving away from the origin of resistor and capacitor, or dummy cell equivalent circuit, the
the corresponding axes. Higher frequency data points are assumedareais1cm .Regardingtheimpedancedatashownin
typically located towards the origin of the plot while lower Fig. 8 for the circuit of Fig. 7, the distance from the origin to
G3 − 89 (2010)
FIG. 7 Equivalent Electrical Circuit Model for a Simple Corroding
Electrode
FIG. 9 Typical Plot for Simple Electrical Model of Fig. 7
8.3.3 In the second type of Bode plot, the negative of the
phase angle,−θ, is plotted on the ordinate and the base ten
logarithm of the frequency is plotted on the abscissa. In this
practiceincreasingvaluesofthenegativeofthephaseangleare
plottedintheverticaldirectionfromtheoriginalongthe yaxis
(ordinate). In this format, a pure capacitive behavior is plotted
FIG. 8 Nyquist Plot for Equivalent Circuit of Fig. 7
as a positive value of 90°. Fig. 10 shows a typical plot for the
simple electrode model shown in Fig. 7.
the first (high frequency) intercept with the abscissa corre-
sponds to R . The distance between the first intercept and the
s
second(lowfrequency)interceptwiththeabscissacorresponds
to R .
p
8.3 Bode Format:
8.3.1 Electrochemical impedance data may be reported as
twotypesofBodeplots.Inthefirstcase,thebasetenlogarithm
of the impedance magnitude or Modulus, |Z|, is plotted on the
ordinate and the base ten logarithm of the frequency is plotted
ontheabscissa.Inthispracticeincreasingfrequencyvaluesare
plotted to the right of the origin parallel to the x axis (abscissa)
and increasing values of impedance magnitude are plotted
vertically from the origin parallel to the y axis (ordinate). The
origin itself is chosen at appropriate nonzero values of imped-
ance magnitude and frequency.
8.3.2 Fig. 9 shows a typical plot for the simple electrical
circuit model of Fig. 7. The magnitude of the high frequency
impedance where the impedance magnitude is independent of
freque
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.