ASTM F1223-03
(Test Method)Standard Test Method for Determination of Total Knee Replacement Constraint
Standard Test Method for Determination of Total Knee Replacement Constraint
SCOPE
1.1 The purpose of this test method is to establish a database of total knee replacement (TKR) motion characteristics with the intent of developing guidelines for the assignment of constraint criteria to TKR designs. (See the Rationale in Appendix X1.)
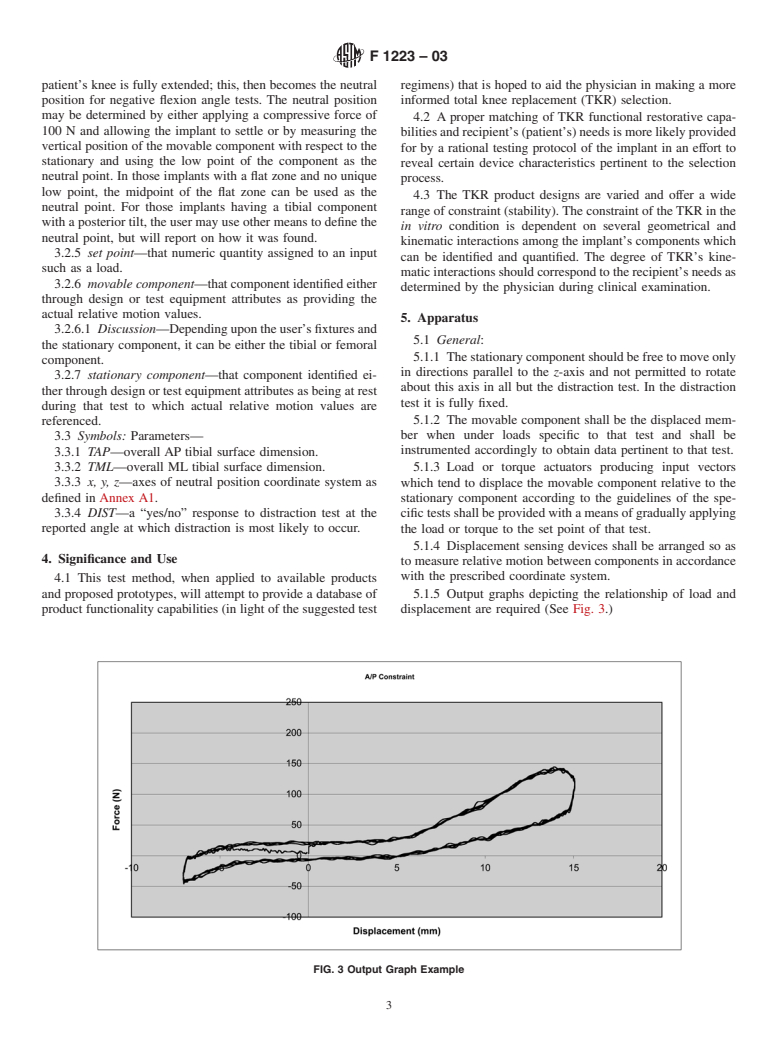
1.2 This test method covers the means by which a TKR constraint may be quantified according to motion delineated by the inherent articular design as determined while under specific loading conditions in an in vitro environment.
1.3 Tests deemed applicable to the constraint determination are antero-posterior draw, medio-lateral shear, rotary laxity, valgus-varus rotation, and distraction, as applicable. Also covered is the identification of geometrical parameters of the contacting surfaces which would influence this motion and the means of reporting the test results. (See Practices E 4.)
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 1223 – 03
Standard Test Method for
1
Determination of Total Knee Replacement Constraint
This standard is issued under the fixed designation F 1223; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (ϵ) indicates an editorial change since the last revision or reapproval.
1. Scope 3.1.2 anterior posterior (AP)—any geometrical length
aligned with the AP orientation.
1.1 Thepurposeofthistestmethodistoestablishadatabase
3.1.3 AP displacement—the relative linear translation be-
of total knee replacement (TKR) motion characteristics with
tween components in the AP direction.
the intent of developing guidelines for the assignment of
3.1.4 AP draw load—the force applied to the movable
constraint criteria to TKR designs. (See the Rationale in
component with its vector aligned in the AP direction causing
Appendix X1.)
or intending to cause an AP displacement.
1.2 This test method covers the means by which a TKR
3.1.5 biconcave—a condylar design with pronounced AP
constraint may be quantified according to motion delineated by
and MLcondylar radii seen as a “dish” in the tibial component
the inherent articular design as determined while under specific
or a “toroid” in the femoral component.
loading conditions in an in vitro environment.
3.1.6 bearing surface—those regions of the component
1.3 Tests deemed applicable to the constraint determination
which are intended to contact its counterpart for load transmis-
are antero-posterior draw, medio-lateral shear, rotary laxity,
sion.
valgus-varus rotation, and distraction, as applicable. Also
3.1.7 condyles—entity designed to emulate the joint
covered is the identification of geometrical parameters of the
anatomy and used as a bearing surface primarily for transmis-
contacting surfaces which would influence this motion and the
sion of the joint reaction force with geometrical properties
means of reporting the test results. (See Practices E 4E4.)
which tend to govern the general kinematics of the TKR.
1.4 This standard does not purport to address all of the
3.1.8 distraction—the separation of the femoral compo-
safety concerns, if any, associated with its use. It is the
nent(s) from the tibial component(s) in the z-direction.
responsibility of the user of this standard to establish appro-
3.1.9 flexion angle—the angulation of the femoral compo-
priate safety and health practices and determine the applica-
nent (about an axis parallel to the y-axis) from the fully
bility of regulatory limitations prior to use.
extended knee position to a position in which a “local” vertical
2. Referenced Documents axis on the component now points posteriorly.
3.1.9.1 Discussion—For many implants, 0° of flexion can
2.1 ASTM Standards:
2
be defined as when the undersurface of the tibial component is
E4 Practices for Force Verification of Testing Machines
3
parallel to the femoral component surface that in vivo contacts
F 2083 Specification for Total Knee Prosthesis
the most distal surface of the femur. This technique may not be
3. Terminology
possibleforsomeimplantsthataredesignedtohaveaposterior
tilt of the tibial component. In these cases, the user shall
3.1 Definitions—Items in this category refer to the geo-
specify how the 0° of flexion position was defined.
metricalandkinematicaspectsofTKRdesignsastheyrelateto
3.1.10 hinge—a mechanical physical coupling between
their human counterparts:
femoral and tibial components which provides a singular axis
3.1.1 anterior curvature—a condylar design which is gen-
about which flexion occurs.
erally planar except for a concave—upward region anteriorly
3.1.11 hyperextension stop—a geometrical feature which
on the tibial component.
arrests further progress of flexion angles of negative value.
3.1.12 internal-external rotation—the relative angulation of
the moveable component about an axis parallel to the z-axis.
1
This test method is under the jurisdiction ofASTM Committee F04 on Medical
3.1.13 joint reaction force—the applied load whose vector
andSurgicalMaterialsandDevices andisthedirectresponsibilityofSubcommittee
F04.15 on Material Test Methods.
is directed parallel to the z-axis, generally considered parallel
Current edition approved Apr. 10, 2003. Published June 2003. Originally
to tibial longitudinal axis.
approved in 1989. Last previous edition approved in 2001 as F 1223 – 01.
2 3.1.14 medio-lateral (ML)—the orientation that is aligned
Annual Book of ASTM Standards, Vol 03.01.
3
Annual Book of ASTM Standards, Vol 13.01. with the y-axis in the defined coordinate system.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
----------------------
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.