ASTM F1926/F1926M-10
(Test Method)Standard Test Method for Evaluation of the Environmental Stability of Calcium Phosphate Granules, Fabricated Forms, and Coatings
Standard Test Method for Evaluation of the Environmental Stability of Calcium Phosphate Granules, Fabricated Forms, and Coatings
SCOPE
1.1 This test method covers calcium phosphate materials intended for use in surgical implant applications.
1.2 Aspects of the biological response to calcium phosphate materials in soft tissue and bone have been reported from laboratory studies and clinical use (1-10).
1.3 The requirements of this specification apply to calcium phosphate materials such as calcium hydroxyapatite (see Specification F1185), beta-tricalcium phosphate (see Specification F1088), and biphasic mixtures thereof with or without intentional addition of other minor components (10 %).
1.4 The material(s) shall be representative of that produced for sale. It shall have been produced and processed under standard manufacturing conditions.
1.5 The materials may be in the form of powders, granules, fabricated forms or coatings; and may be porous, nonporous, textured, and other implantable topographical substrate form representative of the end-use product.
1.6 The calcium phosphate material may constitute the only material in a substrate or it may be one of multiple materials so long as all other materials present do not dissolve under the test conditions described in this test method.
1.7 This test method is limited to the laboratory evaluation of the dissolution rate of a calcium phosphate material. No correlation of the results to in vivo performance is implied.
1.8 The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining values from the two systems may result in non-conformance with the standard.
1.9 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F1926/F1926M − 10
StandardTest Method for
Evaluation of the Environmental Stability of Calcium
1
Phosphate Granules, Fabricated Forms, and Coatings
ThisstandardisissuedunderthefixeddesignationF1926/F1926M;thenumberimmediatelyfollowingthedesignationindicatestheyear
of original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.
A superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.9 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
1.1 This test method covers calcium phosphate materials
responsibility of the user of this standard to establish appro-
intended for use in surgical implant applications.
priate safety and health practices and determine the applica-
1.2 Aspectsofthebiologicalresponsetocalciumphosphate
bility of regulatory limitations prior to use.
materials in soft tissue and bone have been reported from
2
laboratory studies and clinical use (1-10).
2. Referenced Documents
3
1.3 The requirements of this specification apply to calcium
2.1 ASTM Standards:
phosphate materials such as calcium hydroxyapatite (see E691Practice for Conducting an Interlaboratory Study to
Specification F1185), beta-tricalcium phosphate (see Specifi-
Determine the Precision of a Test Method
cation F1088), and biphasic mixtures thereof with or without
F1088Specification for Beta-Tricalcium Phosphate for Sur-
intentional addition of other minor components (<10%).
gical Implantation
F1185Specification for Composition of Hydroxylapatite for
1.4 The material(s) shall be representative of that produced
Surgical Implants
for sale. It shall have been produced and processed under
standard manufacturing conditions.
3. Terminology
1.5 The materials may be in the form of powders, granules,
3.1 Definitions of Terms Specific to This Standard:
fabricated forms or coatings; and may be porous, nonporous,
3.1.1 calcium phosphate—anyoneofanumberofinorganic
textured, and other implantable topographical substrate form
chemicalcompoundscontainingcalciumandphosphateionsas
representative of the end-use product.
its principal constituents.
1.6 The calcium phosphate material may constitute the only
3.1.2 coating—a layer of material mechanically or chemi-
materialinasubstrateoritmaybeoneofmultiplematerialsso
cally adhering to the surface of a substrate.
longasallothermaterialspresentdonotdissolveunderthetest
conditions described in this test method.
4. Dissolution Media
1.7 This test method is limited to the laboratory evaluation
4.1 Water used for preparing reagents or dissolution media
of the dissolution rate of a calcium phosphate material. No
shall be degassed carbon dioxide free deionized or distilled
++
correlation of the results to in vivo performance is implied.
water and have less than 0.1 ppm of residual Ca ion.
1.8 The values stated in either SI units or inch-pound units
4.2 Unbuffered Water Media—Deionized or distilled water
–5 –5 –5
are to be regarded separately as standard. The values stated in
containing8×10 M NaCl,8×10 M CaCl,and5×10
2
each system may not be exact equivalents; therefore, each
MK (PO ).
3 4
system shall be used independently of the other. Combining
4.3 pH 5.5 MES Buffer Media—1.0 M MES, [2-(N-
values from the two systems may result in non-conformance
morphplino)ethanesulfonic acid] having a pH of 5.5 at 37 6
with the standard.
–5 –5
0.5°Candcontaining8×10 MNaCl,8×10 MCaCl ,and
2
–5
5× 10 MK (PO ).
3 4
4.3.1 A buffer concentration of 1.0 M will usually provide
1
This test method is under the jurisdiction ofASTM Committee F04 onMedical
sufficient buffer capacity to keep the solution within 60.1 pH
andSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee
F04.13 on Ceramic Materials.
Current edition approved Dec. 1, 2010. Published December 2010. Originally
3
published in 1998. Last previous edition approved in 2008 as F1926/F1926M–08. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
DOI: 10.1520/F1926_F1926M-10. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
2
The boldface numbers given in parentheses refer to a list of references at the Standards volume information, refer to the standard’s Document Summary page on
end of the text. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F1926/F1926M − 10
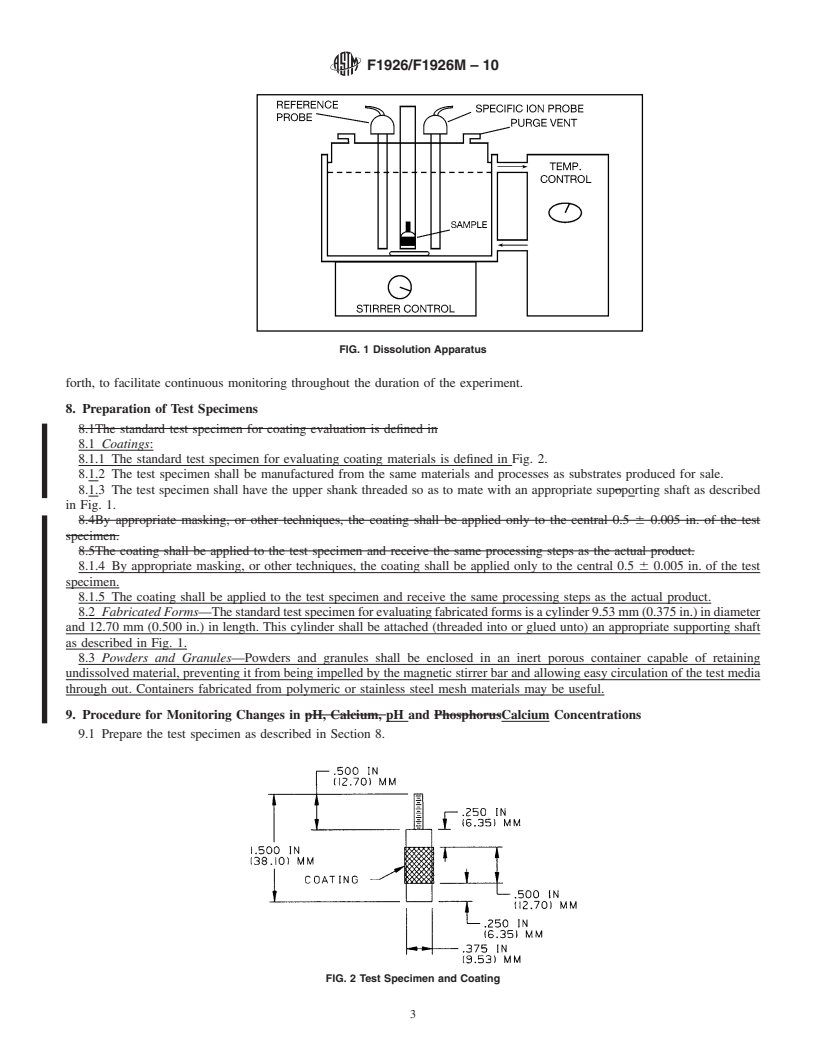
FIG. 1 Dissolution Apparatus
units of the initial value. If this is not the case, the buffer 7. Dissolution Apparatus
capacity should be adjusted accordingly.
7.1 The dissolution vessel shall be of such design to easily
4.3.2 The pH must be adjusted to 5.5 at 37 6 0.5°C
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:F1926–03 Designation: F1926/F1926M – 10
Standard Test Method for
Evaluation of the Environmental Stability of Calcium
1
Phosphate Granules, Fabricated Forms, and Coatings
ThisstandardisissuedunderthefixeddesignationF1926/F1926M;thenumberimmediatelyfollowingthedesignationindicatestheyear
of original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval.
A superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers calcium phosphate coatingsmaterials intended for use in surgical implant applications.
1.2 Aspectsofthebiologicalresponsetocalciumphosphatematerialsinsofttissueandbonehavebeenreportedfromlaboratory
2
studies and clinical use (1-10).
1.3 The requirements of this specification apply to calcium phosphate coatingsmaterials such as calcium hydroxyapatite (see
Specification F1185), beta-tricalcium phosphate (see Specification F1088), and biphasic mixtures thereof with or without
intentional addition of other minor components (<10 %).
1.4The coating(s) shall be representative of that produced for sale. It shall have been produced and processed under standard
manufacturing conditions.
1.5The coatings may be applied to porous, nonporous, textured, and other implantable topographical substrate forms
representative of the end-use product.
1.6The calcium phosphate coating may constitute the only coating on a substrate or be one multiple coatings.
1.7Thistestmethodislimitedtothelaboratoryevaluationofthedissolutionrateofacalciumphosphatecoatings.Nocorrelation
of the results to
1.4 The material(s) shall be representative of that produced for sale. It shall have been produced and processed under standard
manufacturing conditions.
1.5 The materials may be in the form of powders, granules, fabricated forms or coatings; and may be porous, nonporous,
textured, and other implantable topographical substrate form representative of the end-use product.
1.6 The calcium phosphate material may constitute the only material in a substrate or it may be one of multiple materials so
long as all other materials present do not dissolve under the test conditions described in this test method.
1.7 This test method is limited to the laboratory evaluation of the dissolution rate of a calcium phosphate material. No
correlation of the results to in vivo performance is implied.
1.8The values stated in both inch-pound and SI units are to be regarded separately as the standard. The values given in
parentheses are for information only.
1.8 The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each
system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining values from the
two systems may result in non-conformance with the standard.
1.9 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
3
2.1 ASTM Standards:
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
F1088 Specification for Beta-Tricalcium Phosphate for Surgical Implantation
F1185 Specification for Composition of Hydroxylapatite for Surgical Implants
1
ThistestmethodisunderthejurisdictionofASTMCommitteeF04onMedicalandSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommitteeF04.13
on Ceramic Materials.
Current edition approvedApr. 10, 2003. Published May 2003. Originally published in 1998. Last previous edition approved in 1999 as F1926-99. DOI: 10.1520/F1926-03.
Current edition approved Dec. 1, 2010. Published December 2010. Originally published in 1998. Last previous edition approved in 2008 as F1926/F1926M – 08. DOI:
10.1520/F1926_F1926M-10.
2
The boldface numbers given in parentheses refer to a list of references at the end of the text.
3
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
F1926/F1926M – 10
3. Terminolog
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.