ASTM D3803-91(2009)
(Test Method)Standard Test Method for Nuclear-Grade Activated Carbon
Standard Test Method for Nuclear-Grade Activated Carbon
SIGNIFICANCE AND USE
The results of this test method give a conservative estimate of the performance of nuclear-grade activated carbon used in all nuclear power plant HVAC systems for the removal of radioiodine.
SCOPE
1.1 This test method is a very stringent procedure for establishing the capability of new and used activated carbon to remove radio-labeled methyl iodide from air and gas streams. The single test method described is for application to both new and used carbons, and should give test results comparable to those obtained from similar tests required and performed throughout the world. The conditions employed were selected to approximate operating or accident conditions of a nuclear reactor which would severely reduce the performance of activated carbons. Increasing the temperature at which this test is performed generally increases the removal efficiency of the carbon by increasing the rate of chemical and physical absorption and isotopic exchange, that is, increasing the kinetics of the radioiodine removal mechanisms. Decreasing the relative humidity of the test generally increases the efficiency of methyl iodide removal by activated carbon. The water vapor competes with the methyl iodide for adsorption sites on the carbon, and as the amount of water vapor decreases with lower specified relative humidities, the easier it is for the methyl iodide to be adsorbed. Therefore, this test method is a very stringent test of nuclear-grade activated carbon because of the low temperature and high relative humidity specified. This test method is recommended for the qualification of new carbons and the quantification of the degradation of used carbons.
1.1.1 Guidance for testing new and used carbons using conditions different from this test method is offered in Annex A1.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3803 − 91 (Reapproved 2009)
Standard Test Method for
Nuclear-Grade Activated Carbon
This standard is issued under the fixed designation D3803; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method is a very stringent procedure for 2.1 ASTM Standards:
establishing the capability of new and used activated carbon to D1193Specification for Reagent Water
D2652Terminology Relating to Activated Carbon
remove radio-labeled methyl iodide from air and gas streams.
Thesingletestmethoddescribedisforapplicationtobothnew D2854Test Method for Apparent Density of Activated
Carbon
and used carbons, and should give test results comparable to
those obtained from similar tests required and performed E300Practice for Sampling Industrial Chemicals
E691Practice for Conducting an Interlaboratory Study to
throughout the world. The conditions employed were selected
to approximate operating or accident conditions of a nuclear Determine the Precision of a Test Method
2.2 Code of Federal Regulations:
reactor which would severely reduce the performance of
activatedcarbons.Increasingthetemperatureatwhichthistest CFR Title 49,Section 173.34, “Qualification, Maintenance,
is performed generally increases the removal efficiency of the and Use of Cylinders’’
carbon by increasing the rate of chemical and physical absorp- CFR Title 49,Part 178, Subpart C, “Specifications for
tion and isotopic exchange, that is, increasing the kinetics of Cylinders’’
the radioiodine removal mechanisms. Decreasing the relative 2.3 Military Standards:
humidityofthetestgenerallyincreasestheefficiencyofmethyl MIL-F-51068D Filter, Particulate High Efficiency, Fire
iodideremovalbyactivatedcarbon.Thewatervaporcompetes Resistant
with the methyl iodide for adsorption sites on the carbon, and MIL-F-51079A Filter, Medium Fire Resistant, High Effi-
as the amount of water vapor decreases with lower specified ciency
relative humidities, the easier it is for the methyl iodide to be MIL-STD-45662 Calibration Systems Requirements
adsorbed.Therefore, this test method is a very stringent test of 2.4 Other Standards:
nuclear-grade activated carbon because of the low temperature ANSI/ASME N45.2.6 Qualifications of Inspection,
Examination, and Testing Personnel for Nuclear Power
and high relative humidity specified. This test method is
recommended for the qualification of new carbons and the Plants
quantification of the degradation of used carbons.
3. Terminology
1.1.1 Guidance for testing new and used carbons using
3.1 Definitions of Terms Specific to This Standard:
conditions different from this test method is offered in Annex
3.1.1 counter effıciency (CE)—the fraction of the actual
A1.
number of disintegrations of a radioactive sample that is
1.2 The values stated in SI units are to be regarded as
recorded by a nuclear counter.
standard. No other units of measurement are included in this
3.1.2 effıciency (E)—the percentage of the contaminant
standard.
removed from a gas stream by an adsorption bed; expressed
1.3 This standard does not purport to address all of the
mathematically as E = 100 − P, where E and P are given in
safety concerns, if any, associated with its use. It is the
percent.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
bility of regulatory limitations prior to use.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
Published by the General Service Administration, 18th and “F”’ St., N. W.,
This test method is under the jurisdiction of ASTM Committee D28 on Washington, DC 20405.
Activated Carbon and is the direct responsibility of Subcommittee D28.04 on Gas Available from Standardization Documents Order Desk, DODSSP, Bldg. 4,
Phase Evaluation Tests. Section D, 700 Robbins Ave., Philadelphia, PA 19111-5098, http://
Current edition approved April 1, 2009. Published May 2009. Originally dodssp.daps.dla.mil.
approved in 1979. Last previous edition approved in 2004 as D3803–91 (2004). Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
DOI: 10.1520/D3803-91R09. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3803 − 91 (Reapproved 2009)
3.1.3 penetration (P)—the percentage of the contaminant to a depth of 50 6 1 mm, or they may be assembled from two
(CH I) which passes through the equilibrated test bed of separate units each capable of containing carbon to a depth of
standard depth, and is collected on the backup beds during the 25 mm.Two backup canisters, each of 50 6 1 mm total depth,
feed and elution periods under specified conditions. are required. Canisters may be reused after being decontami-
nated to remove residual radioactivity. An acceptable bed
3.1.4 relative humidity (RH)—for the purpose of this test
construction is shown in Fig. 1 with critical dimensions noted.
method, relative humidity is defined as the ratio of the partial
6.2.2 Clamping assemblies are needed for sample and
pressure of water in the gas to the saturation vapor pressure of
backup beds. The only requirements for these assemblies are
water at the gas temperature and pressure. At temperatures
that they provide a smooth sealing face, uniform alignment of
below 100°C, this is the normal definition and relative humid-
bedcanisters,andsufficientclampingforcesothattheleaktest
ity can range from 0 to 100%.
in10.2canbemet.Asuggesteddesignforclampingassemblies
3.2 Definitions—for additional terms relating to this stan-
is shown in Fig. 2.
dard, see Terminology D2652.
6.3 A schematic of a generalized test system is shown in
Fig. 3. This system is designed to operate at approximately
4. Summary of Test Method
30°Cand95%relativehumidity,withagasflowof24.7L/min
4.1 Both new and used carbons are first exposed to humid
at atmospheric pressure. If test conditions which differ signifi-
air (pressure, approximately 1 atm; temperature, 30.0°C; rela-
cantly from these are required, then separate calibrations or
tive humidity, 95%) for a pre-equilibration period of 16 h.
instrumentation, or both, may be required.
Duringthispre-equilibrationperiod,thetestsystemmayberun
unattended with the required parameter monitoring and ad-
equate control devices. Following pre-equilibration, the air
flow is continued for a two-hour equilibration period, during
which the acceptable variability of all parameters is reduced.
The test system must be closely monitored and controlled
during the final four hours of the test. Qualification of
personnel to perform this testing must meet or exceed ANSI/
ASMEN45.2.6—1978,LevelII,whichrequiresacombination
of education and actual test system operation experience.
During the challenge or feed period, radio-labeled methyl
iodideatamassconcentrationof1.75mg/m ofhumidairflow
is passed through the beds for a period of 60 min. Following
the feed period, humid air flow without test adsorbate is
continued at the same conditions for a 60-min elution period.
Throughout the entire test, the effluent from the sample bed
passes through two backup beds containing carbon having a
known high efficiency for methyl iodide.The two backup beds
trap essentially all the radio-labeled methyl iodide that passes
the test bed and provide a differential indication of their
efficiency.At the end of the elution period, the gamma activity
of I in the test and backup beds is measured by a gamma
counter,andthepercentofadsorbatepenetratingthetestbedis
determined.
5. Significance and Use
5.1 The results of this test method give a conservative
estimate of the performance of nuclear-grade activated carbon
used in all nuclear power plant HVAC systems for the removal
of radioiodine.
6. Apparatus
* Standard canister dimension may be used in multiples if desired.
6.1 Sample Preparation Apparatus:
Single test canisters of full depth may be used.
6.1.1 Riffle Sampler, in accordance with 32.5.2 of Practice
1—Bed holder
E300. 2—Adsorption media
3—O-ring gland
6.1.2 Feed Funnel and Vibrator, in accordance with the
4—Perforated screen (both ends)
Procedure Section of Test Method D2854.
5—Retaining snap ring (both ends)
6—Baffle (both ends)
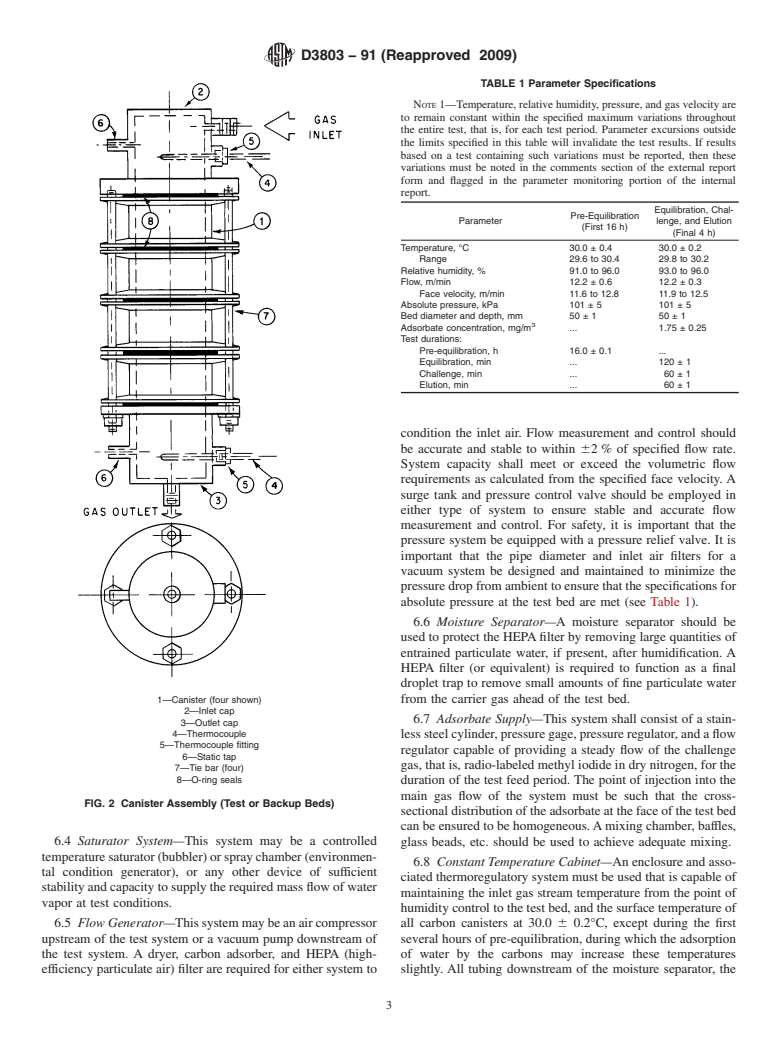
6.2 Sample and Backup Bed Assemblies:
7—Holes for assembly tie-rods (four)
6.2.1 The sample bed canister and backup bed canisters
must each be either a single unit capable of containing carbon FIG. 1 Adsorption Media Test Bed Holder (Canister)
D3803 − 91 (Reapproved 2009)
TABLE 1 Parameter Specifications
NOTE 1—Temperature, relative humidity, pressure, and gas velocity are
to remain constant within the specified maximum variations throughout
the entire test, that is, for each test period. Parameter excursions outside
the limits specified in this table will invalidate the test results. If results
based on a test containing such variations must be reported, then these
variations must be noted in the comments section of the external report
form and flagged in the parameter monitoring portion of the internal
report.
Equilibration, Chal-
Pre-Equilibration
Parameter lenge, and Elution
(First 16 h)
(Final 4 h)
Temperature, °C 30.0 ± 0.4 30.0 ± 0.2
Range 29.6 to 30.4 29.8 to 30.2
Relative humidity, % 91.0 to 96.0 93.0 to 96.0
Flow, m/min 12.2 ± 0.6 12.2 ± 0.3
Face velocity, m/min 11.6 to 12.8 11.9 to 12.5
Absolute pressure, kPa 101 ± 5 101 ± 5
Bed diameter and depth, mm 50 ± 1 50 ± 1
Adsorbate concentration, mg/m . 1.75 ± 0.25
Test durations:
Pre-equilibration, h 16.0 ± 0.1 .
Equilibration, min . 120 ± 1
Challenge, min . 60 ± 1
Elution, min . 60 ± 1
condition the inlet air. Flow measurement and control should
be accurate and stable to within 62% of specified flow rate.
System capacity shall meet or exceed the volumetric flow
requirements as calculated from the specified face velocity. A
surge tank and pressure control valve should be employed in
either type of system to ensure stable and accurate flow
measurement and control. For safety, it is important that the
pressure system be equipped with a pressure relief valve. It is
important that the pipe diameter and inlet air filters for a
vacuum system be designed and maintained to minimize the
pressuredropfromambienttoensurethatthespecificationsfor
absolute pressure at the test bed are met (see Table 1).
6.6 Moisture Separator—A moisture separator should be
used to protect the HEPAfilter by removing large quantities of
entrained particulate water, if present, after humidification. A
HEPA filter (or equivalent) is required to function as a final
droplet trap to remove small amounts of fine particulate water
1—Canister (four shown) from the carrier gas ahead of the test bed.
2—Inlet cap
6.7 Adsorbate Supply—This system shall consist of a stain-
3—Outlet cap
4—Thermocouple
lesssteelcylinder,pressuregage,pressureregulator,andaflow
5—Thermocouple fitting
regulator capable of providing a steady flow of the challenge
6—Static tap
gas,thatis,radio-labeledmethyliodideindrynitrogen,forthe
7—Tie bar (four)
8—O-ring seals
duration of the test feed period. The point of injection into the
main gas flow of the system must be such that the cross-
FIG. 2 Canister Assembly (Test or Backup Beds)
sectionaldistributionoftheadsorbateatthefaceofthetestbed
canbeensuredtobehomogeneous.Amixingchamber,baffles,
6.4 Saturator System—This system may be a controlled glass beads, etc. should be used to achieve adequate mixing.
temperaturesaturator(bubbler)orspraychamber(environmen-
6.8 ConstantTemperatureCabinet—Anenclosureandasso-
tal condition generator), or any other device of sufficient
ciatedthermoregulatorysystemmustbeusedthatiscapableof
stabilityandcapacitytosupplytherequiredmassflowofwater
maintaining the inlet gas stream temperature from the point of
vapor at test conditions.
humiditycontroltothetestbed,andthesurfacetemperatureof
6.5 FlowGenerator—Thissystemmaybeanaircompressor all carbon canisters at 30.0 6 0.2°C, except during the first
upstream of the test system or a vacuum pump downstream of several hours of pre-equilibration, during which the adsorption
the test system. A dryer, carbon adsorber, and HEPA (high- of water by the carbons may increase these temperatures
efficiencyparticulateair)filterarerequiredforeithersystemto slightly. All tubing downstream of the moisture separator, the
D3803 − 91 (Reapproved 2009)
FIG. 3 Schematic of Activated Carbon Test System
carbon bed canisters and holders, temperature and pressure surement system calibration to 60.2°C are required for the
ports and measurement devices upstream and downstream of measurement of test bed inlet air temperature and dew point.
the test bed, and an upstream port and tubing to the dew point The placement of the air temperature RTD must be such that it
sensor all must be included within the temperature controlled isnotsubjecttoradiativeheatingfromthetestbed.Itiscritical
enclosure. In addition, it is highly recommended that a bypass to the exact measurement of relative humidity that the chilled
line be included around the sample bed assembly to avoid mirror RTD and the inlet air temperature RTD be matched
exposing the sample to start-up conditions possibly outside exactly(60.1°C)orthatdifferencesareexactlycorrectedforin
those specified. relative humidity calculations.
6.9 FlowMeasurementandControl—Massflowcontrollers,
6.12 Pressure Measurement Devices—Absolute pressure
control valve and orifice meter, rotameter or any other device
measuring devices must be accurate to within 61% of the
with adequate stability and demonstrated measurement system
reading at standard atmospheric pressure and be capable of
accuracy of 62% of specified flow rate at the test conditions.
digital or analog output to meet the specified recording
All flow measuring devices must use correction factors for
requirements. The sensors and output devices must be cali-
interpretation and application to actual test conditions. These
brated as a unit to ensure system accuracy. The differential
factors must be carefully predetermined and documented. No
pressure device required for measurements across the test bed
flow measuring device should be located directly downstream
mustbecapableofdetectinga0.25kPapressure
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.