ASTM F2582-14
(Test Method)Standard Test Method for Impingement of Acetabular Prostheses
Standard Test Method for Impingement of Acetabular Prostheses
SIGNIFICANCE AND USE
5.1 This test method should be used to evaluate and compare acetabular prostheses to assess the relative degree of constraint for the prosthesis and the damage tolerance under controlled laboratory conditions.
5.2 Although the methodology described attempts to identify physiologically relevant motions and loading conditions, the interpretation of results is limited to an in vitro comparison between acetabular prosthesis designs regarding constraint and their ability to resist impingement fatigue, wear, deformation, and dislocation under the stated test conditions.
SCOPE
1.1 This test method covers a procedure to evaluate acetabular component fatigue, deformation, and wear and femoral head assembly dislocation under dynamic impingement conditions.
1.2 This test method can be used to evaluate single-piece acetabular prostheses, modular prostheses, and constrained prostheses manufactured from polymeric, metallic, or ceramic materials.
1.3 The values stated in SI units are regarded as the standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2582 − 14
Standard Test Method for
1
Impingement of Acetabular Prostheses
This standard is issued under the fixed designation F2582; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope F2091 Specification for Acetabular Prostheses
3
2.2 ISO Standards:
1.1 Thistestmethodcoversaproceduretoevaluateacetabu-
ISO 7206-1 Implants for Surgery – Partial and Total Hip
lar component fatigue, deformation, and wear and femoral
Joint Prostheses – Part 1: Classification and Designation
head assembly dislocation under dynamic impingement condi-
of Dimensions
tions.
ISO 14242-1 Implants for Surgery –Wear ofTotal Hip-Joint
1.2 This test method can be used to evaluate single-piece
Prostheses – Part 1: Loading and Displacement Param-
acetabular prostheses, modular prostheses, and constrained
eters for Wear-Testing Machines and Corresponding En-
prostheses manufactured from polymeric, metallic, or ceramic
vironmental Conditions for Test
materials.
ISO 14242-2 Implants for Surgery –Wear ofTotal Hip-Joint
1.3 The values stated in SI units are regarded as the Prostheses – Part 2: Methods of Measurement
ISO 21535 Non-Active Surgical Implants – Joint Replace-
standard.
ment Implants – Specific Requirements for Hip-Joint
1.4 This standard does not purport to address all of the
Replacement Implants
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
3. Terminology
priate safety and health practices and determine the applica-
3.1 Definitions:
bility of regulatory limitations prior to use.
3.1.1 component separation—the disruption of a connection
between components. May be stable or unstable.
2. Referenced Documents
2 3.1.2 dislocation—the loss of normal physical contact be-
2.1 ASTM Standards:
tween opposing components, usually indicated by large sepa-
E4 Practices for Force Verification of Testing Machines
ration and a loss of stability.
E467 Practice for Verification of Constant Amplitude Dy-
3.1.3 femoral head—convex spherical bearing member for
namic Forces in an Axial Fatigue Testing System
articulation with the natural acetabulum or prosthetic acetabu-
F2003 Practice for Accelerated Aging of Ultra-High Mo-
lum.
lecular Weight Polyethylene after Gamma Irradiation in
Air
3.1.4 impingement—the point at which two opposing com-
F2033 Specification for Total Hip Joint Prosthesis and Hip
ponents collide to restrict motion.
Endoprosthesis Bearing Surfaces Made of Metallic,
3.1.5 joint reaction force—the force directed normal to the
Ceramic, and Polymeric Materials
entry diameter of the acetabular prosthesis (see ISO 7206-1).
3.1.6 locking mechanism—the pieces of various compo-
1
nents that contribute to the fixing of one component to another.
This test method is under the jurisdiction ofASTM Committee F04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
3.1.7 range of motion—the effective pattern of motion
F04.22 on Arthroplasty.
limited by impingement. In one plane this is measured from
Current edition approved April 1, 2014. Published April 2014. Originally
one impingement point to the opposite impingement point.
approved in 2008. Last previous edition approved in 2008 as F2582 – 08. DOI:
10.1520/F2582-14.
3.1.8 subluxation—partial dislocation.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available from International Organization for Standardization (ISO), 1, ch. de
the ASTM website. la Voie-Creuse, CP 56, CH-1211 Geneva 20, Switzerland, http://www.iso.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2582 − 14
4. Summary of Test Method 6.2 Three motion axes shall be capable of controlling and
monitoring angular displacement.
4.1 Acetabular prostheses are evaluated for fatigue,
deformation,andwearunderrepeatedimpingementconditions. 6.3 The equipment may be electromechanical, servo-
Modular acetabular prostheses should be evaluated for addi- hydraulic or other, as long as it meets the requirements of
tional failure mechanisms including separation, loosening, Practices E4 and E467 for force verification.
fracture, and deformation of any component or locking
6.4 The joint reaction force shall be applied through uncon-
mechanism, or both.
strained fixturing that allows for the separation of the
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2582 − 08 F2582 − 14
Standard Test Method for
1
Impingement of Acetabular Prostheses
This standard is issued under the fixed designation F2582; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers a procedure for measuring the range of motion, impingement, and dislocation of a to evaluate
acetabular component fatigue, deformation, and wear and femoral head assembly and acetabular prosthesis.dislocation under
dynamic impingement conditions.
1.2 This test method covers the procedure for static and cyclic fatigue tests.
1.2 This test method maycan be used to evaluate single piece single-piece acetabular prostheses, modular prostheses, and
constrained prostheses manufactured from polymeric, metallic, or ceramic materials.
1.3 The values stated in SI units are regarded as the standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
E4 Practices for Force Verification of Testing Machines
E467 Practice for Verification of Constant Amplitude Dynamic Forces in an Axial Fatigue Testing System
F2003 Practice for Accelerated Aging of Ultra-High Molecular Weight Polyethylene after Gamma Irradiation in Air
F2033 Specification for Total Hip Joint Prosthesis and Hip Endoprosthesis Bearing Surfaces Made of Metallic, Ceramic, and
Polymeric Materials
F2091 Specification for Acetabular Prostheses
3
2.2 ISO Standards:
ISO 7206-1 Implants for Surgery – Partial and Total Hip Joint Prostheses – Part 1: Classification and Designation of Dimensions
ISO 14242-1 Implants for Surgery – Wear of Total Hip-Joint Prostheses – Part 1: Loading and Displacement Parameters for
Wear-Testing Machines and Corresponding Environmental Conditions for Test
ISO 14242-2 Implants for Surgery – Wear of Total Hip-Joint Prostheses – Part 2: Methods of Measurement
ISO 21535 Non-Active Surgical Implants – Joint Replacement Implants – Specific Requirements for Hip-Joint Replacement
Implants
3. Terminology
3.1 Definitions:
3.1.1 component separation—the disruption of a connection between components. May be stable or unstable.
3.1.2 dislocation—the loss of normal physical contact between opposing components, usually indicated by large separation and
a loss of stability.
3.1.3 dislocation moment—the maximum torsional moment (N-m) measured at the point of dislocation. See Fig. 6.
3.1.3 femoral head—convex spherical bearing member for articulation with the natural acetabulum or prosthetic acetabulum.
1
This test method is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.22 on Arthroplasty.
Current edition approved June 1, 2008April 1, 2014. Published July 2008April 2014. Originally approved in 2008. Last previous edition approved in 2008 as F2582 –
08. DOI: 10.1520/F2582-08.10.1520/F2582-14.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from International Organization for Standardization (ISO), 1, ch. de la Voie-Creuse, CP 56, CH-1211 Geneva 20, Switzerland, http://www.iso.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2582 − 14
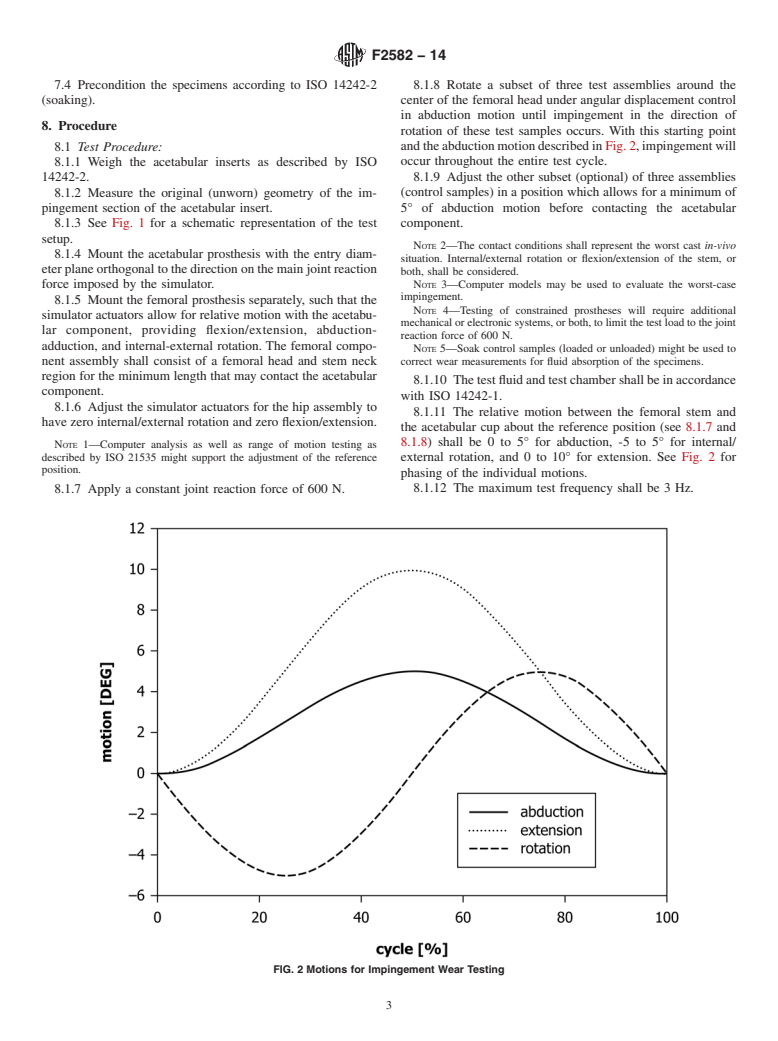
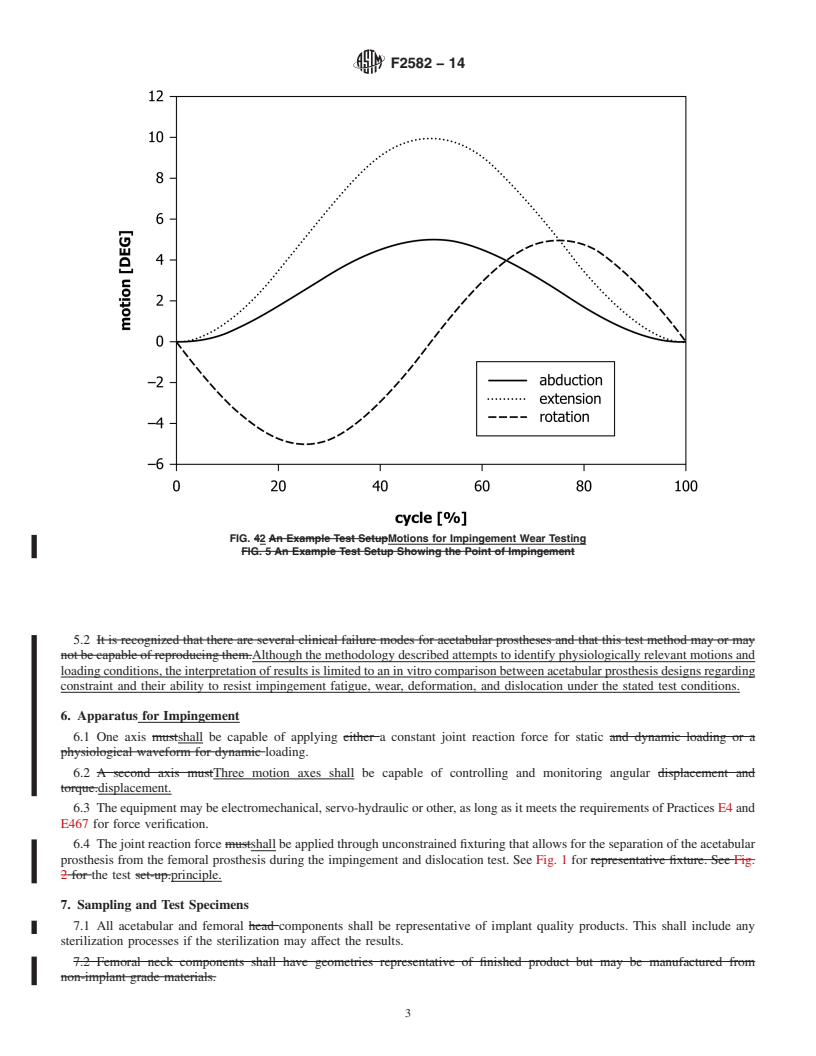
FIG. 2 Schematic Representation of the Test Setup
NOTE 1—The acetabular and femoral prostheses should have freedom to move relative to each other in the plane perpendicular to the joint reaction
force. Flexion-extension (FE), abduction-adduction (AA), and internal-external (IE) rotations are relative motions between the acetabular and femoral
prostheses.
FIG. 31 Schematic Representation Principle of the Test Setup at the PointSet-Up
of Impingement
3.1.4 impingement—the point at which two opposing components collide to restrict motion, usually indicated by a sharp change
in force or moment. See motion.Fig.
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.