ASTM E2810-11
(Practice)Standard Practice for Demonstrating Capability to Comply with the Test for Uniformity of Dosage Units
Standard Practice for Demonstrating Capability to Comply with the Test for Uniformity of Dosage Units
SIGNIFICANCE AND USE
The methodology was originally developed (1-4) for use in drug content uniformity and dissolution but has general application to any multistage test with multiple acceptance criteria. Practice E2709 summarizes the statistical aspects of this methodology. This practice applies the general methodology of Practice E2709 specifically to the UDU test.
While other methods can be used to estimate the probability of passing the UDU test, they are outside the scope of this practice.
The UDU test procedure describes a two-stage sampling test, where at each stage one can pass or continue testing, and the decision to fail is deferred until the second stage. At each stage there are acceptance criteria on the test results as outlined in Table 1.
The UDU test is a market standard. The USP General Notices include the following statement about compendial standards. “The similarity to statistical procedures may seem to suggest an intent to make inference to some larger group of units, but in all cases, statements about whether the compendial standard is met apply only to the units tested.” Therefore, the UDU procedure is not intended for inspecting uniformity of finished product for lot/batch release or as a lot inspection procedure.
The UDU test defines a product requirement to be met at release and throughout the shelf-life of the product.
Passing the UDU test once does not provide statistical assurance that a batch of drug product meets specified statistical quality control criteria.
This practice provides a practical specification that may be applied when uniformity of dosage units is required. An acceptance region for the mean and standard deviation of a set of test results from the lot is defined such that, at a prescribed confidence level, the probability that a future sample from the lot will pass the UDU test is greater than or equal to a prespecified lower probability bound. Having test results fall in the acceptance region provides assurance that a sample wou...
SCOPE
1.1 This practice provides a general procedure for evaluating the capability to comply with the Uniformity of Dosage Units (UDU) test. This test is given in General Chapter 905> Uniformity of Dosage Units of the USP, in 2.9.40 Uniformity of Dosage Units of the Ph. Eur., and in 6.02 Uniformity of Dosage Units of the JP, and these versions are virtually interchangeable. For this multiple-stage test, the procedure computes a lower bound on the probability of passing the UDU test, based on statistical estimates made at a prescribed confidence level from a sample of dosage units.
1.2 This methodology can be used to generate an acceptance limit table, which defines a set of sample means and standard deviations that assures passing the UDU test for a prescribed lower probability bound, confidence level, and sample size.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2810 − 11
StandardPractice for
Demonstrating Capability to Comply with the Test for
Uniformity of Dosage Units
This standard is issued under the fixed designation E2810; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope USP United States Pharmacopeia
1.1 This practice provides a general procedure for evaluat-
3. Terminology
ing the capability to comply with the Uniformity of Dosage
3.1 Definitions—See Terminology E2363 for a more exten-
Units (UDU) test. This test is given in General Chapter <905>
sive listing of terms in ASTM Committee E55 standards.
Uniformity of Dosage Units of the USP, in 2.9.40 Uniformity
of Dosage Units of the Ph. Eur., and in 6.02 Uniformity of
3.2 Definitions of Terms Specific to This Standard:
Dosage Units of the JP, and these versions are virtually
3.2.1 acceptable parameter region, n—the set of values of
interchangeable. For this multiple-stage test, the procedure
parameters characterizing the distribution of test results for
computesalowerboundontheprobabilityofpassingtheUDU
which the probability of passing the lot acceptance procedure
test, based on statistical estimates made at a prescribed
is greater than a prescribed lower bound.
confidence level from a sample of dosage units.
3.2.2 acceptance limit, n—the boundary of the acceptance
1.2 Thismethodologycanbeusedtogenerateanacceptance
region, for example, the maximum sample standard deviation
limit table, which defines a set of sample means and standard
for a given sample mean.
deviations that assures passing the UDU test for a prescribed
3.2.2.1 Discussion—The coefficient of variation (relative
lower probability bound, confidence level, and sample size.
standard deviation) may be substituted for the standard devia-
tion where applicable.
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
3.2.3 acceptance region, n—the set of values of parameter
responsibility of the user of this standard to establish appro-
estimates (that is, sample mean and standard deviation) where
priate safety and health practices and determine the applica-
confidence limits attain a prescribed lower bound on the
bility of regulatory limitations prior to use.
probability of passing a lot acceptance procedure.
3.2.4 confidence level, C, n—the prescribed overall level for
2. Referenced Documents
calculating the uncertainty region of the parameters from the
2.1 ASTM Standards:
sample estimates.
E2363 Terminology Relating to ProcessAnalytical Technol-
3.2.4.1 Discussion—The preset confidence level is stated as
ogy in the Pharmaceutical Industry
a percentage, for example, 100 (1 –α) = 95 %, where α is a
E2709 Practice for Demonstrating Capability to Comply
risk that is allocated to the two parameters being estimated.
with an Acceptance Procedure
3.2.5 lower probability bound, LB, n—thenominalprobabil-
2.2 Other Documents:
3 ity of passing the UDU test for a given set of parameter
JP Japanese Pharmacopoeia
estimates.
Ph. Eur. European Pharmacopoeia
3.2.6 multiple-stage acceptance procedure, n—a procedure
that involves more than one stage of sampling and testing a
This practice is under the jurisdiction of ASTM Committee E55 on Manufac-
given quality characteristic with one or more acceptance
ture of Pharmaceutical Products and is the direct responsibility of Subcommittee
criteria per stage.
E55.03 on General Pharmaceutical Standards.
Current edition approved Oct. 1, 2011. Published December 2011. DOI:
3.2.7 representative sample, n—a sample that consists of a
10.1520/E2810-11.
number of units that are drawn based on rational criteria such
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
as random sampling and intended to assure that the sample
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
accurately portrays the material being sampled
the ASTM website.
Available from the Pharmaceuticals and Medical Devices Agency, Japan,
http://jpdb.nihs.go.jp.
4 5
AvailablefromtheEuropeanCouncil,Strasbourg,France,http://www.edqm.eu. Available from U.S. Pharmacopeia (USP), 12601Twinbrook Pkwy., Rockville,
MD 20852-1790, http://www.usp.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2810 − 11
3.2.8 sampling plan, n—scheme for selecting dosage units the decision to fail is deferred until the second stage. At each
from locations within a batch for testing purposes. stagethereareacceptancecriteriaonthetestresultsasoutlined
3.2.8.1 Discussion—In this standard, a single dosage unit is in Table 1.
selected from each batch location.
4.3 The UDU test is a market standard. The USP General
3.2.9 uniformity of dosage units, UDU, n—the degree of
Notices include the following statement about compendial
uniformity in the amount of the drug substance among dosage
standards.“Thesimilaritytostatisticalproceduresmayseemto
units.
suggest an intent to make inference to some larger group of
3.2.9.1 Discussion—The requirements of the UDU test ap-
units,butinallcases,statementsaboutwhetherthecompendial
ply to each drug substance in dosage units containing one or
standard is met apply only to the units tested.” Therefore, the
more drug substances, unless otherwise specified. The unifor-
UDU procedure is not intended for inspecting uniformity of
mity improves as the variability decreases.
finished product for lot/batch release or as a lot inspection
procedure.
4. Significance and Use
4.3.1 The UDU test defines a product requirement to be met
4.1 The methodology was originally developed (1-4) for
at release and throughout the shelf-life of the product.
use in drug content uniformity and dissolution but has general
4.3.2 Passing the UDU test once does not provide statistical
application to any multistage test with multiple acceptance
assurance that a batch of drug product meets specified statis-
criteria. Practice E2709 summarizes the statistical aspects of
tical quality control criteria.
this methodology. This practice applies the general methodol-
ogy of Practice E2709 specifically to the UDU test.
4.4 This practice provides a practical specification that may
4.1.1 While other methods can be used to estimate the be applied when uniformity of dosage units is required. An
probability of passing the UDU test, they are outside the scope
acceptance region for the mean and standard deviation of a set
of this practice. of test results from the lot is defined such that, at a prescribed
confidence level, the probability that a future sample from the
4.2 TheUDUtestproceduredescribesatwo-stagesampling
lot will pass the UDU test is greater than or equal to a
test, where at each stage one can pass or continue testing, and
prespecifiedlowerprobabilitybound.Havingtestresultsfallin
the acceptance region provides assurance that a sample would
pass the UDU test with at least the specified lower bound
probability. This procedure does not account for any decrease
The boldface numbers in parentheses refer to a list of references at the end of
this standard.
TABLE 1 Uniformity of Dosage Units Test Procedure
NOTE 1—All measurements of dosage units and criteria values are in
percentagelabelclaim(%LC).Ateachstagecalculatethesampleaverage,
X—, and the sample standard deviation, s.
Number
Stage Pass Stage If:
Tested
S 10 |M – X¯|+2.4 s# 15.0, where M
is defined below.
S 20 (1)|M – X¯|+2.0 s# 15.0, using
all 30 results (S +S ).
1 2
(2) No dosage unit is outside the
maximum allowed range of
0.75 * M to 1.25 * M.
M is defined as follows:
If T is less than or equal to 101.5 %LC, and
(1)If X¯ is less than 98.5 %LC,
then M = 98.5 %LC.
(2)If X¯ is between 98.5 and
101.5 %LC, then M = X¯.
(3)If X¯ is greater than
101.5 %LC, then M = 101.5 %LC.
If T is greater than 101.5 %LC, and
(1)If X¯ is less than 98.5 %LC,
then M = 98.5 %LC.
(2)If X¯ is between 98.5 and T,
then M = X¯.
(3)If X¯ is greater than T, then
M = T.
T is the target content per dosage unit at the time of manufacture, ex-
pressed as %LC. Unless otherwise specified in the individual monograph, T
is 100.0 %LC.
E2810 − 11
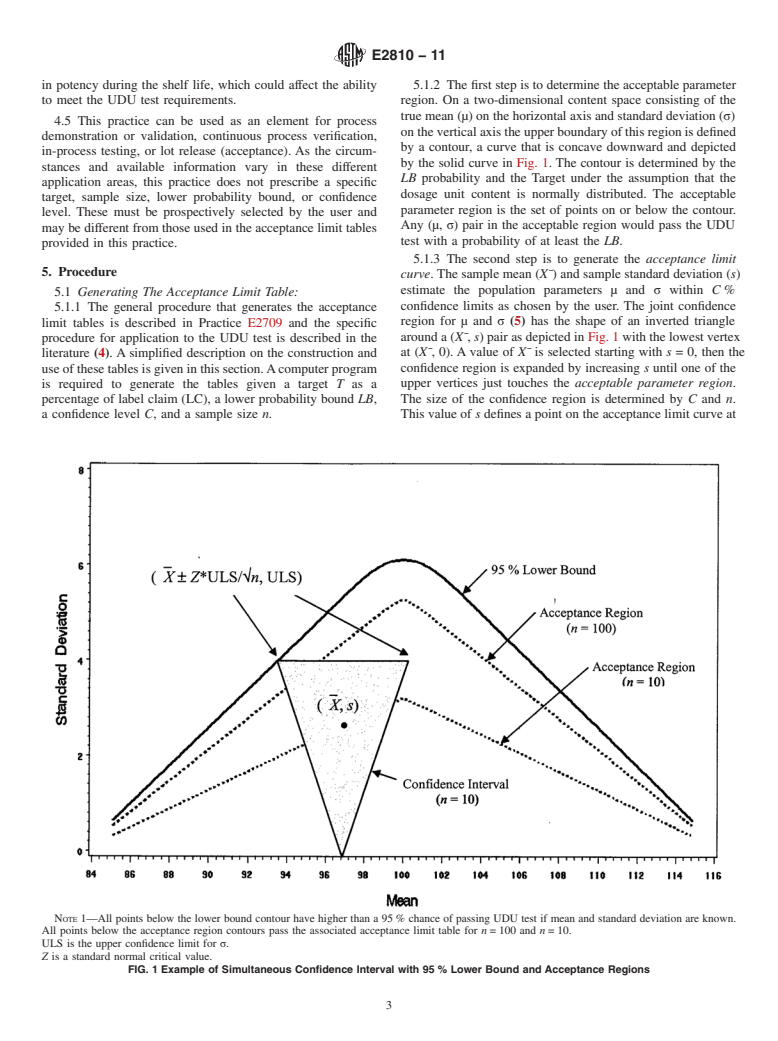
in potency during the shelf life, which could affect the ability 5.1.2 The first step is to determine the acceptable parameter
to meet the UDU test requirements. region. On a two-dimensional content space consisting of the
true mean (µ) on the horizontal axis and standard deviation (σ)
4.5 This practice can be used as an element for process
ontheverticalaxistheupperboundaryofthisregionisdefined
demonstration or validation, continuous process verification,
by a contour, a curve that is concave downward and depicted
in-process testing, or lot release (acceptance). As the circum-
by the solid curve in Fig. 1. The contour is determined by the
stances and available information vary in these different
LB probability and the Target under the assumption that the
application areas, this practice does not prescribe a specific
dosage unit content is normally distributed. The acceptable
target, sample size, lower probability bound, or confidence
parameter region is the set of points on or below the contour.
level. These must be prospectively selected by the user and
Any (µ, σ) pair in the acceptable region would pass the UDU
may be different from those used in the acceptance limit tables
test with a probability of at least the LB.
provided in this practice.
5.1.3 The second step is to generate the acceptance limit
5. Procedure
curve. The sample mean (X¯) and sample standard deviation (s)
estimate the population parameters µ and σ within C %
5.1 Generating The Acceptance Limit Table:
confidence limits as chosen by the user. The joint confidence
5.1.1 The general procedure that generates the acceptance
region for µ and σ (5) has the shape of an inverted triangle
limit tables is described in Practice E2709 and the specific
around a (X¯, s) pair as depicted in Fig. 1 with the lowest vertex
procedure for application to the UDU test is described in the
at (X¯, 0). A value of X¯ is selected starting with s = 0, then the
literature (4). A simplified description on the construction and
use of these tables is given in this section.Acomputer program confidence region is expanded by increasing s until one of the
upper vertices just touches the acceptable parameter region.
is required to generate the tables given a target T as a
percentage of label claim (LC), a lower probability bound LB, The size of the confidence region is determined by C and n.
a confidence level C, and a sample size n. This value of s defines a point on the acceptance limit curve at
NOTE 1—All points below the lower bound contour have higher than a 95 % chance of passing UDU test if mean and standard deviation are known.
All points below the acceptance region contours pass the associated acceptance limit table for n = 100 and n = 10.
ULS is the upper confidence limit for σ.
Z is a standard normal critical value.
FIG. 1 Example of Simultaneous Confidence Interval with 95 % Lower Bound and Acceptance Regions
E2810 − 11
(X¯, s).Additional selections of X¯ then generate the acceptance acceptance limits for standard deviations are symmetrical
limit curve, as depicted as dotted lines in Fig. 1. Acceptance around 100 %LC. This target is also required for interchange-
limit curves are shown for n = 10 and n = 100, illustrating that ability across the ICH regions (6).
theacceptancelimitsapproachtheacceptableparameterregion 5.2.1.1 At the confidence level of C = 95 % used often in
with increasing sample size.
the regulatory arena, three levels of the probability lower
5.1.4 Computer programs have been developed for generat- bound are provided: LB=90%(Table 2), LB=95%(Table 3)
ing acceptance limit tables, but these may not be available to and LB=99%(Table 4).These provide 90 %, 95 %, and 99 %
all practitioners. This practice contains four acceptance limit coverage, respectively, of the population of dosage units under
tables for many practical use situations. consideration. The usual coverage is 95 %.
5.2.1.2 Table 5 is provided at C=90%and LB =95%for
5.2 Using the Acceptance Limit Tables in This Practice:
comparison with Table 3 to demonstrate the effect of a lower
5.2.1 In each table acceptance limits on the standard devia-
confidence level.
tion are given for means ranging 90–110 % of LC in incre-
ments of 0.2 %LC for sample sizes ranging from n=10 to
NOTE 1—Tables can also be generated for other choices for ranges of
means, such as 85.1 to 114.9 %LC, or for other sample sizes.
n = 500. In all tables the target is set at T = 100 %LC, so the
TABLE 2 Acceptance Limits on Sample Standard Deviation (%LC) for
T = 100 %LC,C =95%,LB =90%LC
Sample Average Sample Size (n)
(%LC) 10 30 40 50 60 80 100 120 150 200 500
100.0 2.91 4.36 4.65 4.84 4.99 5.19 5.33 5.43 5.54 5.66 5.93
99.8 or 100.2 2.88 4.31 4.59 4.79 4.94 5.14 5.28 5.38 5.50 5.62 5.91
99.6 or 100.4 2.84 4.26 4.54 4.74 4.89 5.09 5.24 5.34 5.45 5.58 5.88
99.4 or 100.6 2.81 4.21 4.49 4.69 4.83 5.04 5.18 5.29 5.40 5.53 5.84
99.2 or 100.8 2.77 4.16 4.43 4.63 4.77 4.98 5.13 5.23 5.35 5.48 5.79
99.0 or 101.0 2.74 4.10 4.38 4.57 4.72 4.92 5.07 5.17 5.29 5.43 5.74
98.8 or 101.2 2.70 4.05 4.32 4.52 4.66 4.86 5.01 5.11 5.23 5.37 5.69
98.6 or 101.4 2.67 4.00 4.27 4.46 4.60 4.80 4.94 5.05 5.17 5.30 5.63
98.4 or 101.6 2.63 3.95 4.21 4.40 4.54 4.74 4.88 4.99 5.10 5.24 5.56
98.2 or 101.8 2.60 3.89 4.16 4.34 4.48 4.68 4.82 4.92 5.04 5.17 5.49
98.0 or 102.0 2.56 3.84 4.10 4.28 4.42 4.62 4.75 4.86 4.97 5.10 5.43
97.8 or 102.2 2.53 3.79 4.05 4.22 4.36 4.55 4.69 4.79 4.90 5.03 5.35
97.6 or 102.4 2.49 3.74 3.99 4.17 4.30 4.49 4.62 4.72 4.84 4.97 5.28
97.4 or 102.6 2.46 3.68 3.93 4.11 4.24 4.43 4.56 4.66 4.77 4.90 5.21
97.2 or 102.8 2.42 3.63 3.88 4.05 4.18 4.36 4.50 4.59 4.70 4.83 5.13
97.0 or 103.0 2.39 3.58 3.82 3.99 4.12 4.30 4.43 4.53 4.63 4.76 5.06
96.8 or 103.2 2.35 3.53 3.77 3.93 1.06 4.24 4.37 4.46 5.56 4.96 4.99
96.6 or 103.4 2.32 3.48 3.71 3.87 4.00 4.18 4.30 4.39 4.50 4.62 4.91
96.4 or 103.6 2.28 3.42 6.65 3.81 3.94 4.11 4.23 4.33 4.43 4.55 4.84
96.2 or 103.8 2.24 3.37 3.60 3.76 3.88 4.05 4.17 4.26 4.36 4.48 4.77
96.0 or 104.0 2.21 3.32 3.54 3.70 3.82 3.99 4.10 4.19 4.29 4.41 4.69
95.8 or 104.2 2.17 3.26 3.48 3.64 3.76 3.92 4.04 4.13 4.23 4.34 4.62
95.6 or 104.4 2.14 3.21 3.43 3.58 3.70 3.86 3.98 4.06 4.16 4.27 4.54
95.4 or 104.6 2.1
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.