ASTM F564-00
(Specification)Standard Specification and Test Methods for Metallic Bone Staples
Standard Specification and Test Methods for Metallic Bone Staples
SCOPE
1.1 This specification covers characterization of the design and mechanical function of metallic staples used in the internal fixation of the muscular skeletal system. It is not the intention of this specification to describe or specify specific designs for metallic bone staples.
1.2 This specification includes the following four test methods for measuring mechanical properties of metallic bone staples:
1.2.1 Test Method for Constant Amplitude Bending Fatigue Tests of Metallic Bone Staples—Annex A1.
1.2.2 Test Method for Pull-Out Fixation Strength of Metallic Bone Staples—Annex A2.
1.2.3 Test Method for Soft Tissue Fixation Strength of Metallic Bone Staples—Annex A3.
1.2.4 Test Method for Elastic Static Bending of Metallic Bone Staples—Annex A4.
1.3 Unless otherwise indicated, the values stated in SI units are to be regarded as standard. The values given in parentheses are given for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 564 – 00
Standard Specification and Test Methods for
Metallic Bone Staples
This standard is issued under the fixed designation F 564; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope E 4 Practices for Force Verification of Testing Machines
E 122 Practice for Choice of Sample Size to Estimate a
1.1 This specification covers characterization of the design
Measure of Quality for a Lot or Process
and mechanical function of metallic staples used in the internal
E 467 Practice for Verification of Constant Amplitude Dy-
fixation of the muscular skeletal system. It is not the intention
namic Loads in an Axial Load Fatigue Testing Machine
of this specification to describe or specify specific designs for
F 75 Specification for Cast Cobalt-Chromium-Molybdenum
metallic bone staples.
Alloy for Surgical Implant Applications
1.2 This specification includes the following four test meth-
F 86 Practice for Surface Preparation and Marking of Me-
ods for measuring mechanical properties of metallic bone
tallic Surgical Implants
staples:
F 382 Test Method for Static Bending Properties of Metallic
1.2.1 Test Method for Constant Amplitude Bending Fatigue
Bone Plates
Tests of Metallic Bone Staples—Annex A1.
F 565 Standard Practice for Care and Handling of Orthope-
1.2.2 Test Method for Pull-Out Fixation Strength of Metal-
dic Implants and Instruments
lic Bone Staples—Annex A2.
F 601 Practice for Fluorescent Penetrant Inspection of Me-
1.2.3 Test Method for Soft Tissue Fixation Strength of
tallic Surgical Implants
Metallic Bone Staples—Annex A3.
F 629 Practice for Radiography of Cast Metallic Surgical
1.2.4 Test Method for Elastic Static Bending of Metallic
Implants
Bone Staples—Annex A4.
1.3 Unless otherwise indicated, the values stated in SI units
3. Finish
are to be regarded as standard. The values given in parentheses
3.1 Staples conforming to this specification shall be finished
are given for information only.
and identified in accordance with Practice F 86, as appropriate.
1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
4. Inspection Practices
responsibility of the user of this standard to establish appro-
4.1 Staples made in accordance with Specification F 75
priate safety and health practices and determine the applica-
should be inspected in accordance with Practice F 601 or
bility of regulatory limitations prior to use.
X-rayed in accordance with Practice F 629.
2. Referenced Documents
5. Care and Handling
2.1 ASTM Standards:
5.1 Staples should be cared for and handled in accordance
with F 565, as appropriate.
6. Keywords
6.1 bending test; bone fixation; fatigue test; fixation devices;
metallic bone staples; orthopaedic medical devices; pullout
test; soft tissue fixation; surgical implants
This specification is under the jurisdiction of ASTM Committee F-04 on
Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.21 on Osteosynthesis. Annual Book of ASTM Standards, Vol 03.01.
Current edition approved May 10, 2000. Published August 2000. Originally Annual Book of ASTM Standards, Vol 14.02.
e1 4
published as F 564 – 85. Last previous edition F 564 – 85 (1997) . Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
F 564
ANNEXES
(Mandatory Information)
A1. TEST METHOD FOR CONSTANT AMPLITUDE BENDING FATIGUE TESTS OF METALLIC BONE STAPLES
A1.1 Scope staple is fixed securely in the block using a moldable filling or
grouting agent. The extension design should minimize the
A1.1.1 This test method covers procedures for the perfor-
weight to reduce the influence on the staple while maintaining
mance of constant amplitude fatigue testing of metallic staples
sufficient stiffness to transfer the load to the staple without
used in internal fixation of the musculoskeletal system. This
undesirable deflection. Holes for pin and clevis fixation are
test method may be used when testing in air at ambient
optional (see Figs. A1.1-A1.3).
temperature or in an aqueous or physiological solution.
A1.1.2 The values stated in SI units are to be regarded as the
NOTE A1.1—Variations in fixation hole configuration may be required
standard. for staple legs with noncircular cross sections. Also, it is necessary to
provide a gap between the underside of the staple bridge and edge of the
A1.1.3 This standard does not purport to address all of the
staple extender in most cases. This is necessary to eliminate contact
safety concerns, if any, associated with its use. It is the
between the staple bridge (or other bridge features such as tissue spikes)
responsibility of the user of this standard to establish appro-
and the staple extender. However, this gap should be standardized within
priate safety and health practices and determine the applica-
any test group as required.
bility of regulatory limitations prior to use.
A1.4.2.2 4-Point Bend Fixture—A standard or modified
A1.2 Summary of Test Method
bending fixture that produces pure bending in the staple
without appreciable shear or torsion when used to apply load to
A1.2.1 Metallic bone staples are tested under bending loads
the staple through the staple extensions.
until the specimen fails or a predetermined number of cycles
A1.4.2.3 Pin and Clevice Fixture—A standard or modified
has been applied to it. Bending tests may be performed in one
fixture used to apply a distractive or compressive load to the
of two modes: either pure, in-plane bending; or tension (or
staple through the staple extensions to produce bending in the
compression) combined with in-plane bending. Tests using
staple similar to that seen in vivo.
either of these methods may be conducted at ambient condi-
A1.4.3 Filling or Grouting Agent—A stiff, moldable filler,
tions or in aqueous or physiological solutions (at either room
such as epoxy, acrylic cement, or a low-melting point alloy (for
temperature or 37°C).
example, Wood’s metal) used to secure the staple leg within the
A1.3 Significance and Use
staple extension.
A1.3.1 This test method is used to determine the fatigue
A1.4.4 Aqueous Solution—Tap water, distilled water, physi-
resistance of metallic bone staples when subjected to repetitive ological saline, or similar aqueous solutions, used to immerse
loading for large numbers of cycles. This information may also
the test specimens fully during the test.
be useful for comparing the effect of variations in staple A1.4.5 Constant Temperature Bath—An aqueous bath ca-
material, geometry, surface condition, or placement under
pable of maintaining the samples and containers at physiologic
certain circumstances.
A1.3.2 It is essential that uniform fatigue practices be
established in order that such basic fatigue data be comparable
and reproducible and can be correlated among laboratories.
A1.3.3 The results of fatigue tests are suitable for direct
application to design only when the service conditions parallel
the test conditions exactly. This test method may not be
appropriate for all types of bone staple applications. The user
is cautioned to consider the appropriateness of the test method
in view of the materials being tested and their potential
application.
A1.4 Apparatus
A1.4.1 Testing Machines, conforming to the requirements of
Practices E 4 and E 467. The loads used for determining
strengths shall be within the loading range of the testing
machine as defined in Practices E 4 and E 467.
A1.4.2 Gripping Devices:
A1.4.2.1 Staple Extensions—Pairs of specially designed
metal blocks that permit the holding of individual staples for
the application of bending fatigue loads. The legs of each staple
are fitted into fixation holes in each block with minimal
clearance to restrict bending of the staple within the hole. The FIG. A1.1 4-Point Bending of Staples in Extension
F 564
be round, square, or polygonal in cross section, and they may
possess serrations or barbs to increase the fixation or purchase
strength in the bone.
A1.5.3 Staple Bridge—The cross member of the staple
connecting the legs; the bridge may be smooth or possess
spikes or projections on the underside for the retention of soft
tissue or other material.
A1.6 Procedure
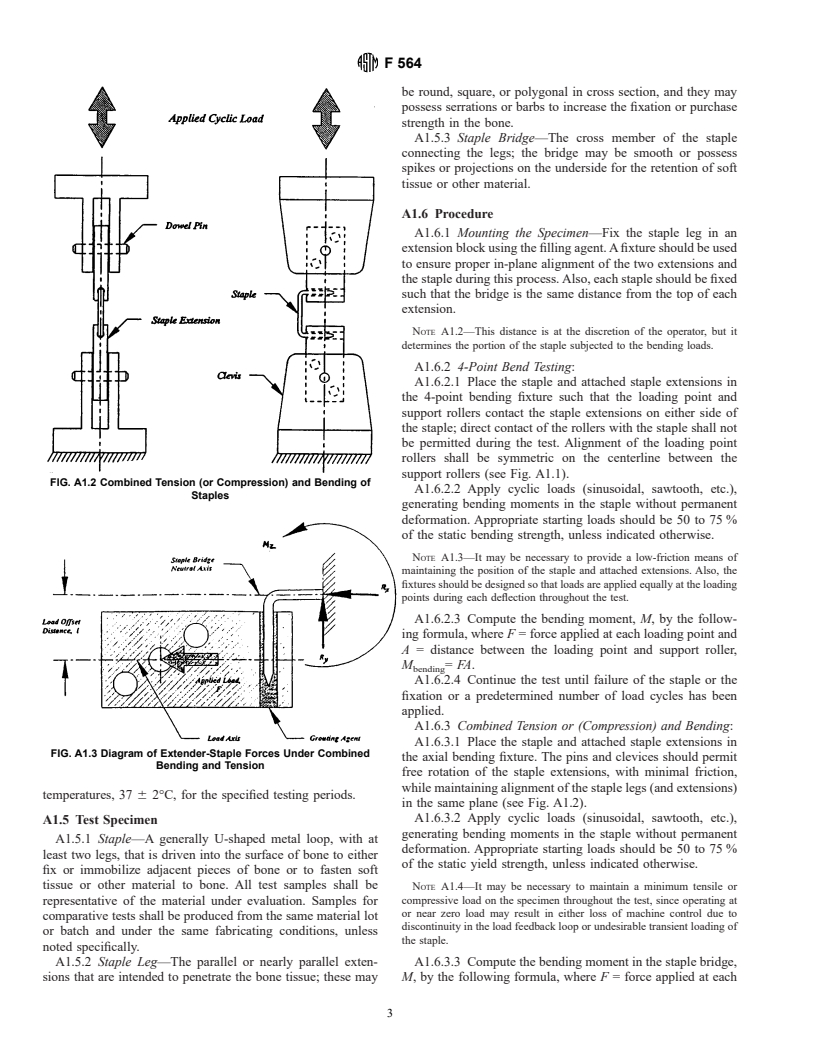
A1.6.1 Mounting the Specimen—Fix the staple leg in an
extension block using the filling agent. A fixture should be used
to ensure proper in-plane alignment of the two extensions and
the staple during this process. Also, each staple should be fixed
such that the bridge is the same distance from the top of each
extension.
NOTE A1.2—This distance is at the discretion of the operator, but it
determines the portion of the staple subjected to the bending loads.
A1.6.2 4-Point Bend Testing:
A1.6.2.1 Place the staple and attached staple extensions in
the 4-point bending fixture such that the loading point and
support rollers contact the staple extensions on either side of
the staple; direct contact of the rollers with the staple shall not
be permitted during the test. Alignment of the loading point
rollers shall be symmetric on the centerline between the
support rollers (see Fig. A1.1).
FIG. A1.2 Combined Tension (or Compression) and Bending of
A1.6.2.2 Apply cyclic loads (sinusoidal, sawtooth, etc.),
Staples
generating bending moments in the staple without permanent
deformation. Appropriate starting loads should be 50 to 75 %
of the static bending strength, unless indicated otherwise.
NOTE A1.3—It may be necessary to provide a low-friction means of
maintaining the position of the staple and attached extensions. Also, the
fixtures should be designed so that loads are applied equally at the loading
points during each deflection throughout the test.
A1.6.2.3 Compute the bending moment, M, by the follow-
ing formula, where F = force applied at each loading point and
A = distance between the loading point and support roller,
M = FA.
bending
A1.6.2.4 Continue the test until failure of the staple or the
fixation or a predetermined number of load cycles has been
applied.
A1.6.3 Combined Tension or (Compression) and Bending:
A1.6.3.1 Place the staple and attached staple extensions in
FIG. A1.3 Diagram of Extender-Staple Forces Under Combined
the axial bending fixture. The pins and clevices should permit
Bending and Tension
free rotation of the staple extensions, with minimal friction,
while maintaining alignment of the staple legs (and extensions)
temperatures, 37 6 2°C, for the specified testing periods.
in the same plane (see Fig. A1.2).
A1.6.3.2 Apply cyclic loads (sinusoidal, sawtooth, etc.),
A1.5 Test Specimen
generating bending moments in the staple without permanent
A1.5.1 Staple—A generally U-shaped metal loop, with at
deformation. Appropriate starting loads should be 50 to 75 %
least two legs, that is driven into the surface of bone to either
of the static yield strength, unless indicated otherwise.
fix or immobilize adjacent pieces of bone or to fasten soft
tissue or other material to bone. All test samples shall be NOTE A1.4—It may be necessary to maintain a minimum tensile or
compressive load on the specimen throughout the test, since operating at
representative of the material under evaluation. Samples for
or near zero load may result in either loss of machine control due to
comparative tests shall be produced from the same material lot
discontinuity in the load feedback loop or undesirable transient loading of
or batch and under the same fabricating conditions, unless
the staple.
noted specifically.
A1.5.2 Staple Leg—The parallel or nearly parallel exten- A1.6.3.3 Compute the bending moment in the staple bridge,
sions that are intended to penetrate the bone tissue; these may M, by the following formula, where F = force applied at each
F 564
center of each pin and L = distance between the load applica- lot number, and dimensions (including leg length, bridge
tion axis, that is, the pin center, and the neutral axis of the width, and length), as appropriate.
staple bridge, M = FL (see Fig. A1.3). A1.8.1.2 Test Type—4-point or combined tension (or com-
bending
pression) and bending.
NOTE A1.5—The application of this test method produces bending,
A1.8.1.3 Fixation Geometry—Load point separation dis-
tensile (or compressive), and shear stresses in the staple. The direction and
tances (4-point bending), load offset distance (combined ten-
magnitudes of these stresses should be analyzed using superposition
sion and bending), staple bridge-extension distance, and so
theory or other suitable methods.
forth.
A1.6.3.4 Continue the test until failure of the staple or the
A1.8.1.4 Minimum and maximum cycle loads, test fre-
fixation or a predetermined number of load cycles has been
quency (for example, cycles/s), and forcing function type (sine,
applied.
ramp, saw tooth, and so forth).
A1.6.4 Stress Verification—It is recommended that strain
A1.8.1.5 Bending moment, M (N-m).
gages (or extensometry) be used to measure the bending strains
A1.8.1.6 Load ratio, R, where R = minimum load/maximum
induced in the specimen. This is accomplished most easily on
load.
the staple bridge, but it may be possible to perform on a portion
A1.8.1.7 Test Environment—Ambient air or physiological
of the staple leg or at the leg-bridge junction under certain
solution.
circumstances and with certain staple designs. The recom-
A1.8.1.8 Number of cycles at failure or test termination
mended technique is to strain gage the actual fatigue test
(runout).
specimens, if possible, provided that the installation of strain
A1.8.1.9 Location of fatigue fracture (if applicable).
gage will not influence the test results.
A1.8.1.10 Reason for test termination, that is, staple failure,
A1.7 Test Termination fixation failure, runout to specified cycle limit, etc.
A1.7.1 Continue the tests until the specimen fails or a
A1.9 Precision
predetermined number of cycles has been applied to the
A1.9.1 Intralaboratory and interlaboratory reproducibility
specimen. Failure should be defined as complete separation, a
have not been determined systematically.
crack visible at a specified magnification, a crack of certain
A1.10 Rationale (Nonmandatory Information)
dimensions, or by some other criterion. State the criterion
selected for defining failure when reporting the results. A1.10.1 This test method is intended to aid in characterizing
the fatigue behavior of metallic bone staples used for the
A1.7.2 A test shall be cons
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.