ASTM F1717-04
(Test Method)Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model
Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model
SIGNIFICANCE AND USE
Spinal implants are generally composed of several components which, when connected together, form a spinal implant assembly. Spinal implant assemblies are designed to provide some stability to the spine while arthrodesis takes place. These test methods outline standard materials and methods for the evaluation of different spinal implant assemblies so that comparison between different designs may be facilitated.
These test methods are used to quantify the static and dynamic mechanical characteristics of different designs of spinal implant assemblies. The mechanical tests are conducted in vitro using simplified load schemes and do not attempt to mimic the complex loads of the spine.
The loads applied to the spinal implant assemblies in vivo will, in general, differ from the loading configurations used in these test methods. The results obtained here cannot be used directly to predict in vivo performance. The results can be used to compare different component designs in terms of the relative mechanical parameters.
Fatigue testing in a simulated body fluid or saline may cause fretting, corrosion, or lubricate the interconnections and thereby affect the relative performance of tested devices. This test should be initially performed dry (ambient room conditions) for consistency. The effect of environment may be significant. Repeating all or part of these test methods in simulated body fluid, saline (9 g NaCl per 1000 mL water), a saline drip, water, or a lubricant should be considered. The maximum recommended frequency for this type of cyclic testing should be 5 Hz.
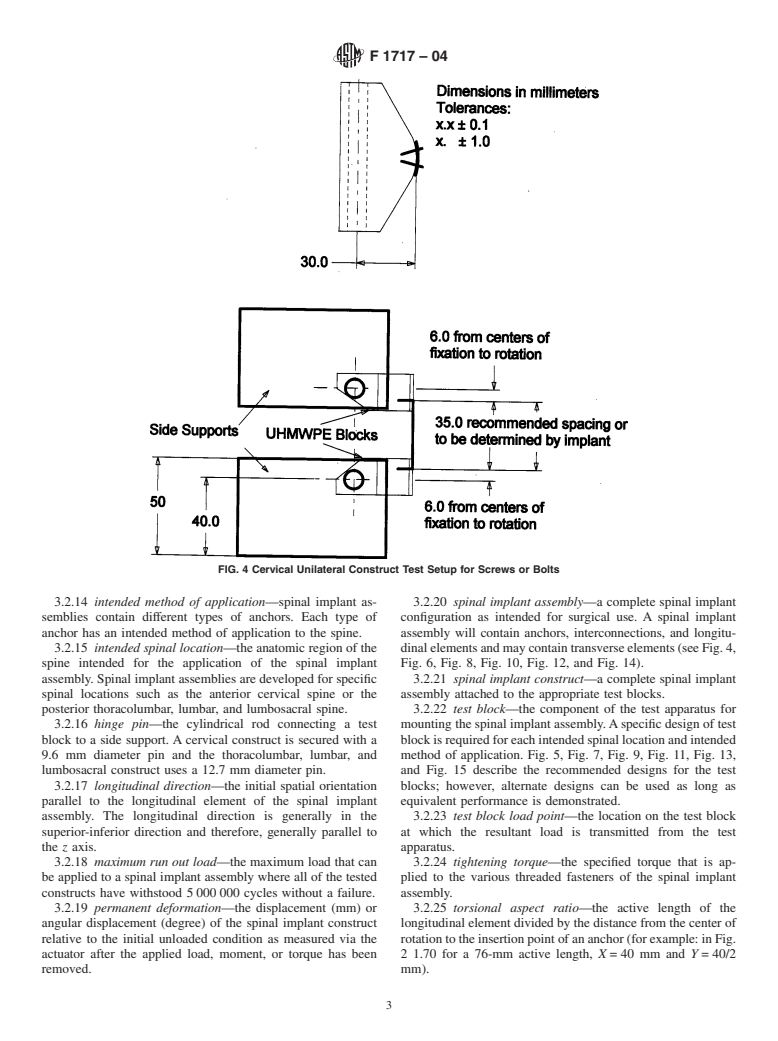
The location of the longitudinal elements is determined by where the anchors are clinically placed against bony structures. The perpendicular distance to the load direction (block moment arm) between the axis of a hinge pin and the anchor’attachment-points to a UHMWPE block is independent of anchor-type. The distance between the anchor’attachment point to the UHMWPE block and the center of the longitud...
SCOPE
1.1 These test methods cover the materials and methods for the static and fatigue testing of spinal implant assemblies in a vertebrectomy model. The test materials for most combinations of spinal implant components can be specific depending on the intended spinal location and intended method of application to the spine.
1.2 These test methods are intended to provide a basis for the mechanical comparison among past, present, and future spinal implant assemblies. They allow comparison of spinal implant constructs with different intended spinal locations and methods of application to the spine. These test methods are not intended to define levels of performance, since sufficient knowledge is not available to predict the consequences of the use of a particular device.
1.3 These test methods set out guidelines for load types and methods of applying loads. Methods for three static load types and one fatigue test are defined for the comparative evaluation of spinal implant assemblies.
1.4 These test methods establish guidelines for measuring displacements, determining the yield load, and evaluating the stiffness and strength of the spinal implant assembly.
1.5 Some spinal constructs may not be testable in all test configurations.
1.6 Values stated in SI units are to be regarded as standard.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F1717–04
Standard Test Methods for
1
Spinal Implant Constructs in a Vertebrectomy Model
This standard is issued under the fixed designation F1717; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope E6 Terminology Relating to Methods of Mechanical Test-
ing
1.1 These test methods cover the materials and methods for
E739 Practice for Statistical Analysis of Linear or Linear-
the static and fatigue testing of spinal implant assemblies in a
ized Stress-Life (S-N) and Strain-Life (e-N) Fatigue Data
vertebrectomymodel.Thetestmaterialsformostcombinations
3
E1150 Definitions of Terms Relating to Fatigue
ofspinalimplantcomponentscanbespecificdependingonthe
F1582 Terminology Relating to Spinal Implants
intended spinal location and intended method of application to
F2077 Test Methods For Intervertebral Body Fusion De-
the spine.
vices
1.2 These test methods are intended to provide a basis for
the mechanical comparison among past, present, and future
3. Terminology
spinal implant assemblies. They allow comparison of spinal
3.1 Definitions:
implant constructs with different intended spinal locations and
3.1.1 For definitions of terms relating to these test methods,
methodsofapplicationtothespine.Thesetestmethodsarenot
see Terminology E6, Terminology F1582, and Definitions
intended to define levels of performance, since sufficient
E1150.
knowledge is not available to predict the consequences of the
3.2 Definitions of Terms Specific to This Standard:
use of a particular device.
3.2.1 active length of the longitudinal element—the straight
1.3 These test methods set out guidelines for load types and
line distance between the center of attachment of the superior
methods of applying loads. Methods for three static load types
anchor and the center of attachment of the inferior anchor.
and one fatigue test are defined for the comparative evaluation
3.2.2 angular displacement at 2 % offset yield (degrees)—
of spinal implant assemblies.
the angular displacement of a construct measured via the
1.4 These test methods establish guidelines for measuring
actuatorthatproducesapermanentangulardisplacementinthe
displacements, determining the yield load, and evaluating the
X-Y plane equal to 0.020 times the torsional aspect ratio (see
stiffness and strength of the spinal implant assembly.
Point A in Fig. 1).
1.5 Some spinal constructs may not be testable in all test
3.2.3 block moment arm—the perpendicular to the applied
configurations.
load between the insertion point of an anchor and the axis of
1.6 Values stated in SI units are to be regarded as standard.
the hinge pin.
1.7 This standard does not purport to address all of the
3.2.4 compressive or tensile bending stiffness (N/mm)—the
safety concerns, if any, associated with its use. It is the
compressive or tensile bending yield force divided by elastic
responsibility of the user of this standard to establish appro-
displacement (see the initial slope of line BC in Fig. 1).
priate safety and health practices and determine the applica-
3.2.5 compressive or tensile bending ultimate load (N)—the
bility of regulatory limitations prior to use.
maximum compressive or tensile force in X-Z plane applied to
2. Referenced Documents a spinal implant assembly (see the force at Point E in Fig. 1).
2
Theultimateloadshouldbeafunctionofthedeviceandnotof
2.1 ASTM Standards:
the load cell or testing machine.
D638 Test Method for Tensile Properties of Plastic
3.2.6 compressive or tensile bending yield load (N)—the
E4 Practices for Force Verification of Testing Machines
compressive or tensile bending force in X-Z plane necessary to
produce a permanent deformation equal to 0.020 times the
activelengthofthelongitudinalelement(seetheforceatPoint
1
These test methods are under the jurisdiction of ASTM Committee F04 on
D in Fig. 1).
Medical and Surgical Materials and Devices and are the direct responsibility of
3.2.7 coordinate system/axes—three orthogonal axes are
Subcommittee F04.25 on Spinal Devices.
Current edition approved Apr. 1, 2004. Published April 2004. Originally defined in Fig. 2 and Fig. 3. The anterior-posterior axis is X
approved in 1996. Last previous edition approved in 2001 as F1717–01.
with positive being anterior. The medial-lateral axis is Y with
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
3
the ASTM website. Withdrawn.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Consho
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.