ASTM D5317-98(2017)
(Test Method)Standard Test Method for Determination of Chlorinated Organic Acid Compounds in Water by Gas Chromatography with an Electron Capture Detector
Standard Test Method for Determination of Chlorinated Organic Acid Compounds in Water by Gas Chromatography with an Electron Capture Detector
SIGNIFICANCE AND USE
5.1 Chlorinated phenoxyacid herbicides, and other organic acids are used extensively for weed control. Esters and salts of 2,4-D and silvex have been used as aquatic herbicides in lakes, streams, and irrigation canals. Phenoxy acid herbicides can be toxic even at low concentrations. For example, the 96 h, TLm for silvex is 2.4 mg/L for bluegills (1).8 These reasons make apparent the need for a standard test method for such compounds in water.
SCOPE
1.1 This test method covers a gas chromatographic procedure for the quantitative determination of selected chlorinated acids and other acidic herbicides in water. Similar chemicals may also be determined by this test method, but it is the user's responsibility to verify the applicability of this test method to any compounds not listed in this scope. The acid form of the following compounds were interlaboratory tested using this test method, and the results were found acceptable:2
Analyte
Chemical Abstract Services
Registry Number
Bentazon
25057-89-0
2,4-D
94-75-7
2,4-DB
94-82-6
DCPA acid metabolites 2
Dicamba
1918-00-9
3,5-Dichlorobenzoic acid
51-36-5
Dichlorprop
120-36-5
5-Hydroxydicamba
7600-50-2
Pentachlorophenol (PCP)
87-86-5
Picloram
1918-02-1
2,4,5-T
93-76-5
2,4,5-TP (Silvex)
93-72-1
1.2 This test method may be applicable to the determination of salts and esters of analyte compounds. The form of each acid is not distinguished by this test method. Results are calculated and reported for each listed analyte as the total free acid.
1.3 This test method has been validated in an interlaboratory test for reagent water and finished tap water. The analyst should recognize that precision and bias reported in Section 18 may not be applicable to other waters.
1.4 This test method is restricted to use by or under the supervision of analysts experienced in the use of gas chromatography (GC) and in the interpretation of gas chromatograms. Each analyst must demonstrate the ability to generate acceptable results with this test method using the procedure described in 19.3. It is the user's responsibility to ensure the validity of this test method for waters of untested matrices.
1.5 Analytes that are not separated chromatographically, that is, which have very similar retention times, cannot be individually identified and measured in the same calibration mixture or water sample unless an alternate technique for identification and quantitation exists (16.6, 16.7, and 16.8).
1.6 When this test method is used to analyze unfamiliar samples for any or all of the analytes given in 1.1, analyte identifications must be confirmed by at least one additional qualitative technique.
1.7 The values stated in SI units are to be regarded as standard. The values given in parentheses are mathematical conversions to inch-pound units that are provided for information only and are not considered standard.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific warning statements, see Sections 6, 8, 9, and 10.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D5317 − 98 (Reapproved 2017)
Standard Test Method for
Determination of Chlorinated Organic Acid Compounds in
Water by Gas Chromatography with an Electron Capture
Detector
This standard is issued under the fixed designation D5317; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ableresultswiththistestmethodusingtheproceduredescribed
in 19.3. It is the user’s responsibility to ensure the validity of
1.1 This test method covers a gas chromatographic proce-
this test method for waters of untested matrices.
dure for the quantitative determination of selected chlorinated
acids and other acidic herbicides in water. Similar chemicals 1.5 Analytes that are not separated chromatographically,
may also be determined by this test method, but it is the user’s that is, which have very similar retention times, cannot be
responsibility to verify the applicability of this test method to individually identified and measured in the same calibration
any compounds not listed in this scope. The acid form of the mixture or water sample unless an alternate technique for
following compounds were interlaboratory tested using this identification and quantitation exists (16.6, 16.7, and 16.8).
test method, and the results were found acceptable:
1.6 When this test method is used to analyze unfamiliar
Chemical Abstract Services
samples for any or all of the analytes given in 1.1, analyte
Analyte
Registry Number
identifications must be confirmed by at least one additional
Bentazon 25057-89-0
qualitative technique.
2,4-D 94-75-7
2,4-DB 94-82-6
1.7 The values stated in SI units are to be regarded as
DCPA acid metabolites
Dicamba 1918-00-9 standard. The values given in parentheses are mathematical
3,5-Dichlorobenzoic acid 51-36-5
conversions to inch-pound units that are provided for informa-
Dichlorprop 120-36-5
tion only and are not considered standard.
5-Hydroxydicamba 7600-50-2
Pentachlorophenol (PCP) 87-86-5
1.8 This standard does not purport to address all of the
Picloram 1918-02-1
safety concerns, if any, associated with its use. It is the
2,4,5-T 93-76-5
2,4,5-TP (Silvex) 93-72-1
responsibility of the user of this standard to establish appro-
priate safety, health, and environmental practices and deter-
1.2 This test method may be applicable to the determination
mine the applicability of regulatory limitations prior to use.
ofsaltsandestersofanalytecompounds.Theformofeachacid
For specific warning statements, see Sections 6, 8, 9, and 10.
is not distinguished by this test method. Results are calculated
1.9 This international standard was developed in accor-
and reported for each listed analyte as the total free acid.
dance with internationally recognized principles on standard-
1.3 Thistestmethodhasbeenvalidatedinaninterlaboratory
ization established in the Decision on Principles for the
testforreagentwaterandfinishedtapwater.Theanalystshould
Development of International Standards, Guides and Recom-
recognize that precision and bias reported in Section 18 may
mendations issued by the World Trade Organization Technical
not be applicable to other waters.
Barriers to Trade (TBT) Committee.
1.4 This test method is restricted to use by or under the
supervision of analysts experienced in the use of gas chroma-
2. Referenced Documents
tography (GC) and in the interpretation of gas chromatograms. 3
2.1 ASTM Standards:
Each analyst must demonstrate the ability to generate accept-
D1129 Terminology Relating to Water
D1193 Specification for Reagent Water
D2777 Practice for Determination of Precision and Bias of
This test method is under the jurisdiction of ASTM Committee D19 on Water
Applicable Test Methods of Committee D19 on Water
andisthedirectresponsibilityofSubcommitteeD19.06onMethodsforAnalysisfor
Organic Substances in Water.
Current edition approved Dec. 15, 2017. Published January 2018. Originally
approved in 1992. Last previous edition approved in 2011 as D5317 – 93 (2011). For referenced ASTM standards, visit the ASTM website, www.astm.org, or
DOI: 10.1520/D5317-98R17. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
DCPA monoacid and diacid metabolites are included in the scope of this test Standards volume information, refer to the standard’s Document Summary page on
method; DCPA diacid metabolite is used for validation studies. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5317 − 98 (2017)
D3370 Practices for Sampling Water from Closed Conduits 4.2 This test method provides a magnesium silicate
D3856 Guide for Management Systems in Laboratories cleanupproceduretoaidintheeliminationofinterferencesthat
Engaged in Analysis of Water may be present.
D4210 Practice for Intralaboratory Quality Control Proce-
5. Significance and Use
dures and a Discussion on Reporting Low-Level Data
5.1 Chlorinated phenoxyacid herbicides, and other organic
(Withdrawn 2002)
acids are used extensively for weed control. Esters and salts of
D5789 Practice for Writing Quality Control Specifications
2,4-D and silvex have been used as aquatic herbicides in lakes,
for Standard Test Methods for Organic Constituents
streams, and irrigation canals. Phenoxy acid herbicides can be
(Withdrawn 2002)
toxic even at low concentrations. For example, the 96 h, TL
m
2.2 EPA Standard:
for silvex is 2.4 mg/L for bluegills (1). These reasons make
Method 515.1 Revision 4.0, Methods for the Determination
apparent the need for a standard test method for such com-
of Organic Compounds in Drinking Water
pounds in water.
2.3 OSHA Standard:
29 CFR 1910 OSHA Safety and Health Standards, General 6. Interferences
Industry
6.1 Method interferences may be caused by contaminants in
solvents, reagents, glassware and other sample processing
3. Terminology
apparatus that lead to discrete artifacts or elevated baselines in
3.1 Definitions:
gas chromatograms. All reagents and apparatus must be rou-
3.1.1 For definitions of terms used in this standard, refer to
tinely demonstrated to be free from interferences under the
Terminology D1129.
conditions of the analysis by running laboratory reagent blanks
as described in 19.2.
3.2 Definitions of Terms Specific to This Standard:
6.1.1 Glassware must be scrupulously cleaned (2). Clean all
3.2.1 internal standard, n—a pure analyte(s) added to a
glassware as soon as possible after use by thoroughly rinsing
solution in known amount(s) and used to measure the relative
with the last solvent used in it. Follow by washing with hot
responses of other method analytes and surrogates that are
water and detergent and thoroughly rinsing with dilute acid,
components of the same solution.
tap, and reagent water. Drain dry, and heat in an oven or muffle
3.2.1.1 Discussion—The internal standard must be an ana-
furnace at 400°C for 1 h. Do not heat volumetric ware.
lyte that is not a sample component.
Thorough rinsing with acetone may be substituted for the
3.2.2 surrogate analyte, n—a pure analyte(s), which is
heating. After drying and cooling, seal and store glassware in
extremely unlikely to be found in any sample, and which is
a clean environment to prevent any accumulation of dust or
added to a sample aliquot in known amount(s) before extrac-
other contaminants. Store inverted or capped with aluminum
tionandismeasuredwiththesameproceduresusedtomeasure
foil. Thermally stable materials such as PCBs may not be
other sample components.
eliminated by this treatment.
3.2.2.1 Discussion—Thepurposeofasurrogateanalyteisto
6.1.2 The use of high purity reagents and solvents helps to
monitor method performance with each sample.
minimize interference problems. Purification of solvents by
distillation in all-glass systems may be required. (Warning—
4. Summary of Test Method
When a solvent is purified, stabilizers added by the manufac-
4.1 The compounds listed in 1.1, in water samples, are
turer are removed, thus potentially making the solvent hazard-
converted into sodium salts by adjusting the pH to 12 with
ous. Also, when a solvent is purified, preservatives added by
sodium hydroxide solution (240 g/L) and shaking for 1 h.
the manufacturer are removed, thus potentially reducing the
Extraneous neutral material is removed by extraction with
shelf-life.)
methylene chloride. The sample is acidified, the acids are
6.2 The acid forms of the analytes are strong organic acids
extracted with ethyl ether and converted to methyl esters using
that react readily with alkaline substances and can be lost
diazomethane.After the excess reagent is removed, the methyl
during sample preparation. Glassware and glass wool must be
estersaredeterminedbycapillarycolumnGCusinganelectron
acid-rinsed with hydrochloric acid (1 + 9) and the sodium
capture (EC) detector. Other detection systems, such as micro-
sulfatemustbeacidifiedwithsulfuricacidpriortousetoavoid
coulometric and electrolytic conductivity, are not as sensitive
analyte loses due to adsorption.
as EC for measurement of chlorinated acid esters but are more
specific and less subject to interferences. A mass spectrometer
6.3 Organic acids and phenols, especially chlorinated
may also be used as a detector. compounds, cause the most direct interference with the deter-
mination.Alkaline hydrolysis and subsequent extraction of the
basic sample removes many chlorinated hydrocarbons and
The last approved version of this historical standard is referenced on
phthalateestersthatmightotherwiseinterferewiththeelectron
www.astm.org.
capture analysis.
AvailablefromUnitedStatesEnvironmentalProtectionAgency(EPA),William
Jefferson Clinton Bldg., 1200 Pennsylvania Ave., NW, Washington, DC 20460,
http://www.epa.gov. Florisil, a trademark of, and available from, Floridin Co., 2 Gateway Center,
Available from U.S. Government Printing Office, Superintendent of Pittsburgh, PA15222, or its equivalent, has been found satisfactory for this purpose.
Documents, 732 N. Capitol St., NW, Washington, DC 20401-0001, http:// The boldface numbers in parentheses refer to the list of references at the end of
www.access.gpo.gov. this test method.
D5317 − 98 (2017)
6.4 Interferences by phthalate esters can pose a major preparation. If this is not the case, chromatographic compara-
problem in pesticide analysis when using the ECD. These bility of standards to sample may be affected.
compounds generally appear in the chromatogram as large
7. Apparatus and Equipment
peaks. Common flexible plastics contain varying amounts of
phthalates, which are easily extracted or leached during labo-
7.1 Sample Bottle—Borosilicate amber, 1-L volume with
ratory operations. Cross contamination of clean glassware
graduations, fitted with screw caps lined with TFE-
routinely occurs when plastics are handled during extraction
fluorocarbon. Protect samples from light. The container must
steps, especially when solvent-wetted surfaces are handled.
be washed and dried as described in 6.1.1 before use to
Interferences from phthalates can best be minimized by avoid-
minimize contamination. Cap liners are cut to fit from sheets
ingtheuseofplasticsinthelaboratory.Exhaustivepurification
and extracted with methanol overnight prior to use.
of reagents and glassware may be required to eliminate
7.2 Glassware.
background phthalate contamination (3).
7.2.1 Separatory funnel, 2000-mL, with TFE-fluorocarbon
6.5 Interfering contamination may occur when a sample
stopcocks, ground glass or TFE-fluorocarbon stoppers.
containing low concentrations of analytes is analyzed imme-
7.2.2 Tumbler bottle, 1.7-L with TFE-fluorocarbon lined
diately following a sample containing relatively high concen-
screw cap. Cap liners are cut to fit from sheets and extracted
trations of analytes. Between-sample rinsing of the sample
with methanol overnight prior to use.
syringe and associated equipment with methyl-t-butyl-ether
7.2.3 Concentrator tube, Kuderna-Danish (K-D), 10 or
(MTBE) can minimize sample cross contamination. After
25-mL, graduated. Calibration must be checked at the volumes
analysis of a sample containing high concentrations of
employed in the procedure. Ground-glass stoppers are used to
analytes, one or more injections of MTBE should be made to
prevent evaporation of extracts.
ensure that accurate values are obtained for the next sample.
7.2.4 Evaporative flask, K-D, 500-mL.Attach to concentra-
tor tube with springs.
6.6 Matrix interferences may be caused by contaminants
7.2.5 Snyder column, K-D, three ball macro.
that are coextracted from the sample. Also, note that all
7.2.6 Snyder column, K-D, two ball micro.
analytes listed in Table 1 are not resolved from each other on
7.2.7 Flask, round bottom, 500-mLwith 24/40 ground glass
any one column, that is, one analyte of interest may be an
joint.
interferent for another analyte of interest. The extent of matrix
7.2.8 Vials, glass, 5 to 10-mL capacity with TFE-
interferences will vary considerably from source to source,
fluorocarbon lined screw cap.
depending upon the water sampled. The procedures in Section
16 can be used to overcome many of these interferences.
7.3 Boiling Stone, TFE-fluorocarbon.
Positive identifications should be confirmed. See 16.6, 16.7,
7.4 Water Bath, heated, capable of temperature control
and 16.8.
(62°C). The bath should be used in a hood.
6.7 It is important that samples and working standards be
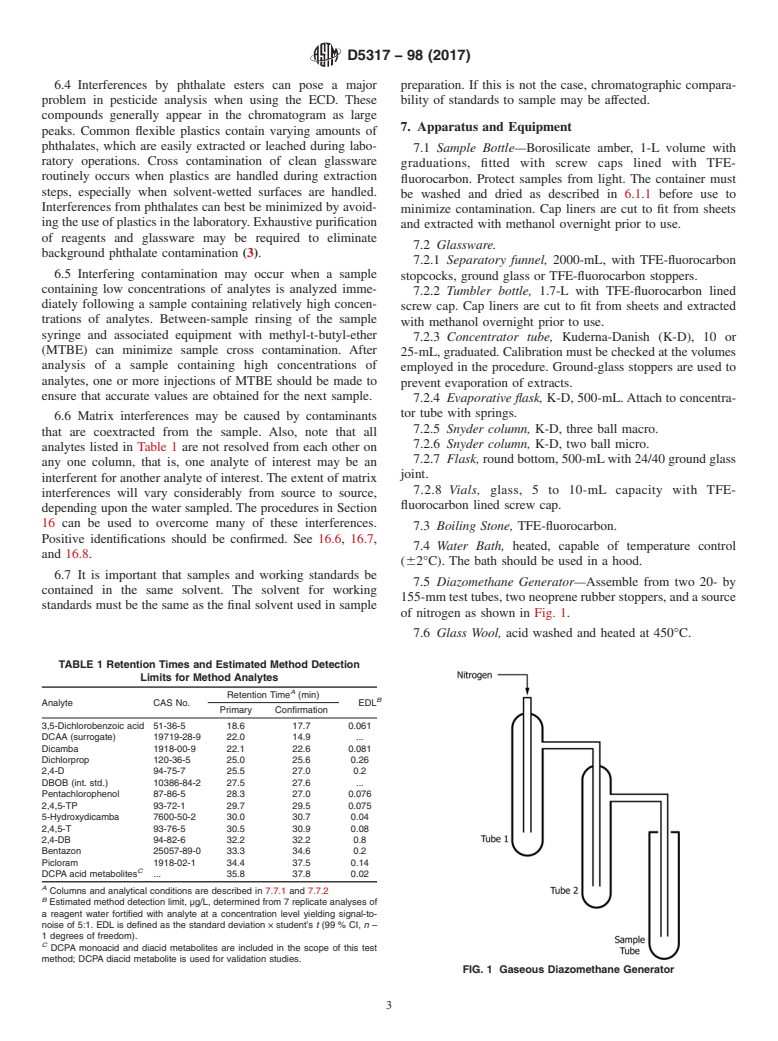
7.5 Diazomethane Generator—Assemble from two 20- by
contained in the same solvent. The solvent for working
155-mm test tubes, two neoprene rubber stoppers, and a source
standards must be the same as the final solvent used in sample
of nitrogen as shown in Fig. 1.
7.6 Glass Wool, acid washed and heated at 450°C.
TABLE 1 Retention Times and Estimated Method Detection
Limits for Method Analytes
A
Retention Time (min)
B
Analyte CAS No. EDL
Primary Confirmation
3,5-Dichlorobenzoic acid 51-36-5 18.6 17.7 0.061
DCAA (surrogate) 19719-28-9 22.0 14.9 .
Dicamba 1918-00-9 22.1 22.6 0.081
Dichlorprop 120-36-5 25.0 25.6 0.26
2,4-D 94-75-7 25.5 27.0 0.2
DBOB (int. std.) 10386-84-2 27.5 27.6 .
Pentachlorophenol 87-86-5 28.3 27.0 0.076
2,4,5-TP 93-72-1 29.7 29.5 0.075
5-Hydroxydicamba 7600-50-2 30.0 30.7 0.04
2,4,5-T 93-76-5 30.5 30.9 0.08
2,4-DB 94-82-6 32.2 32.2 0.8
Bentazon 25057-89-0 33.3 34.6 0.2
Picloram 1918-02-1 34.4 37.5 0.14
C
DCPA acid metabolites . 35.8 37.8 0.02
A
Columns and analytical conditions are described in 7.7.1 and 7.7.2
B
Estimated method detection limit, µg/L, determined from 7 replicate analys
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D5317 − 98 (Reapproved 2011) D5317 − 98 (Reapproved 2017)
Standard Test Method for
Determination of Chlorinated Organic Acid Compounds in
Water by Gas Chromatography with an Electron Capture
Detector
This standard is issued under the fixed designation D5317; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers a gas chromatographic procedure for the quantitative determination of selected chlorinated acids
and other acidic herbicides in water. Similar chemicals may also be determined by this test method, but it is the user’s responsibility
to verify the applicability of this test method to any compounds not listed in this scope. The acid form of the following compounds
were interlaboratory tested using this test method, and the results were found acceptable:
Chemical Abstract Services
Analyte
Registry Number
Analyte Chemical Abstract Services
Registry Number
Bentazon 25057-89-0
2,4-D 94-75-7
2,4-DB 94-82-6
DCPA acid metabolites
Dicamba 1918-00-9
3,5-Dichlorobenzoic acid 51-36-5
Dichlorprop 120-36-5
5-Hydroxydicamba 7600-50-2
Pentachlorophenol (PCP) 87-86-5
Picloram 1918-02-1
2,4,5-T 93-76-5
2,4,5-TP (Silvex) 93-72-1
1.2 This test method may be applicable to the determination of salts and esters of analyte compounds. The form of each acid
is not distinguished by this test method. Results are calculated and reported for each listed analyte as the total free acid.
1.3 This test method has been validated in an interlaboratory test for reagent water and finished tap water. The analyst should
recognize that precision and bias reported in Section 18 may not be applicable to other waters.
1.4 This test method is restricted to use by or under the supervision of analysts experienced in the use of gas chromatography
(GC) and in the interpretation of gas chromatograms. Each analyst must demonstrate the ability to generate acceptable results with
this test method using the procedure described in 19.3. It is the user’s responsibility to ensure the validity of this test method for
waters of untested matrices.
1.5 Analytes that are not separated chromatographically, that is, which have very similar retention times, cannot be individually
identified and measured in the same calibration mixture or water sample unless an alternate technique for identification and
quantitation exists (16.6, 16.7, and 16.8).
1.6 When this test method is used to analyze unfamiliar samples for any or all of the analytes given in 1.1, analyte identifications
must be confirmed by at least one additional qualitative technique.
1.7 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information
only.mathematical conversions to inch-pound units that are provided for information only and are not considered standard.
This test method is under the jurisdiction of ASTM Committee D19 on Water and is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for
Organic Substances in Water.
Current edition approved May 1, 2011Dec. 15, 2017. Published June 2011January 2018. Originally approved in 1992. Last previous edition approved in 20032011 as
ε1
D5317 – 93 (2003)(2011). . DOI: 10.1520/D5317-98R11.10.1520/D5317-98R17.
DCPA monoacid and diacid metabolites are included in the scope of this test method; DCPA diacid metabolite is used for validation studies.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5317 − 98 (2017)
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use. For specific warning statements, see Sections 6, 8, 9, and 10.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
D1129 Terminology Relating to Water
D1193 Specification for Reagent Water
D2777 Practice for Determination of Precision and Bias of Applicable Test Methods of Committee D19 on Water
D3370 Practices for Sampling Water from Closed Conduits
D3856 Guide for Management Systems in Laboratories Engaged in Analysis of Water
D4210 Practice for Intralaboratory Quality Control Procedures and a Discussion on Reporting Low-Level Data (Withdrawn
2002)
D5789 Practice for Writing Quality Control Specifications for Standard Test Methods for Organic Constituents (Withdrawn
2002)
2.2 EPA Standard:
Method 515.1, Method 515.1 Revision 4.0, Methods for the Determination of Organic Compounds in Drinking Water
2.3 OSHA Standard:
29 CFR 1910 OSHA Safety and Health Standards, General Industry
3. Terminology
3.1 Definitions—Definitions: For definitions of terms used in this test method, refer to Terminology D1129.
3.1.1 For definitions of terms used in this standard, refer to Terminology D1129.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 internal standard—standard, n—a pure analyte(s) added to a solution in known amount(s) and used to measure the relative
responses of other method analytes and surrogates that are components of the same solution.
3.2.1.1 Discussion—
The internal standard must be an analyte that is not a sample component.
3.2.2 surrogate analyte—analyte, n—a pure analyte(s), which is extremely unlikely to be found in any sample, and which is
added to a sample aliquot in known amount(s) before extraction and is measured with the same procedures used to measure other
sample components.
3.2.2.1 Discussion—
The purpose of a surrogate analyte is to monitor method performance with each sample.
4. Summary of Test Method
4.1 The compounds listed in 1.1, in water samples, are converted into sodium salts by adjusting the pH to 12 with sodium
hydroxide solution (240 g/L) and shaking for 1 h. Extraneous neutral material is removed by extraction with methylene chloride.
The sample is acidified, the acids are extracted with ethyl ether and converted to methyl esters using diazomethane. After the excess
reagent is removed, the methyl esters are determined by capillary column GC using an electron capture (EC) detector. Other
detection systems, such as microcoulometric and electrolytic conductivity, are not as sensitive as EC for measurement of
chlorinated acid esters but are more specific and less subject to interferences. A mass spectrometer may also be used as a detector.
4.2 This test method provides a magnesium silicate cleanup procedure to aid in the elimination of interferences that may be
present.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
The last approved version of this historical standard is referenced on www.astm.org.
EPA/600/4-88/039, 1989, available from Environmental Monitoring Systems Laboratory, U.S. Environmental Protection Agency, Cincinnati, OH 45268.Available from
United States Environmental Protection Agency (EPA), William Jefferson Clinton Bldg., 1200 Pennsylvania Ave., NW, Washington, DC 20460, http://www.epa.gov.
Available from U.S. Government Printing Office, Superintendent of Documents, 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401.20401-0001,
http://www.access.gpo.gov.
Florisil, a trademark of, and available from, Floridin Co., 2 Gateway Center, Pittsburgh, PA 15222, or its equivalent, has been found satisfactory for this purpose.
D5317 − 98 (2017)
5. Significance and Use
5.1 Chlorinated phenoxyacid herbicides, and other organic acids are used extensively for weed control. Esters and salts of 2,4-D
and silvex have been used as aquatic herbicides in lakes, streams, and irrigation canals. Phenoxy acid herbicides can be toxic even
at low concentrations. For example, the 96 h, TL for silvex is 2.4 mg/L for bluegills (1)). . These reasons make apparent the need
m
for a standard test method for such compounds in water.
6. Interferences
6.1 Method interferences may be caused by contaminants in solvents, reagents, glassware and other sample processing
apparatus that lead to discrete artifacts or elevated baselines in gas chromatograms. All reagents and apparatus must be routinely
demonstrated to be free from interferences under the conditions of the analysis by running laboratory reagent blanks as described
in 19.2.
6.1.1 Glassware must be scrupulously cleaned (2). Clean all glassware as soon as possible after use by thoroughly rinsing with
the last solvent used in it. Follow by washing with hot water and detergent and thoroughly rinsing with dilute acid, tap, and reagent
water. Drain dry, and heat in an oven or muffle furnace at 400°C for 1 h. Do not heat volumetric ware. Thorough rinsing with
acetone may be substituted for the heating. After drying and cooling, seal and store glassware in a clean environment to prevent
any accumulation of dust or other contaminants. Store inverted or capped with aluminum foil. Thermally stable materials such as
PCBs may not be eliminated by this treatment.
6.1.2 The use of high purity reagents and solvents helps to minimize interference problems. Purification of solvents by
distillation in all-glass systems may be required. (Warning—When a solvent is purified, stabilizers added by the manufacturer are
removed, thus potentially making the solvent hazardous. Also, when a solvent is purified, preservatives added by the manufacturer
are removed, thus potentially reducing the shelf-life. Warning—) When a solvent is purified, stabilizers added by the manufacturer
are removed, thus potentially making the solvent hazardous. Also, when a solvent is purified, preservatives added by the
manufacturer are removed, thus potentially reducing the shelf-life.)
6.2 The acid forms of the analytes are strong organic acids that react readily with alkaline substances and can be lost during
sample preparation. Glassware and glass wool must be acid-rinsed with hydrochloric acid (1 + 9) and the sodium sulfate must be
acidified with sulfuric acid prior to use to avoid analyte loses due to adsorption.
6.3 Organic acids and phenols, especially chlorinated compounds, cause the most direct interference with the determination.
Alkaline hydrolysis and subsequent extraction of the basic sample removes many chlorinated hydrocarbons and phthalate esters
that might otherwise interfere with the electron capture analysis.
6.4 Interferences by phthalate esters can pose a major problem in pesticide analysis when using the ECD. These compounds
generally appear in the chromatogram as large peaks. Common flexible plastics contain varying amounts of phthalates, which are
easily extracted or leached during laboratory operations. Cross contamination of clean glassware routinely occurs when plastics
are handled during extraction steps, especially when solvent-wetted surfaces are handled. Interferences from phthalates can best
be minimized by avoiding the use of plastics in the laboratory. Exhaustive purification of reagents and glassware may be required
to eliminate background phthalate contamination (3).
6.5 Interfering contamination may occur when a sample containing low concentrations of analytes is analyzed immediately
following a sample containing relatively high concentrations of analytes. Between-sample rinsing of the sample syringe and
associated equipment with methyl-t-butyl-ether (MTBE) can minimize sample cross contamination. After analysis of a sample
containing high concentrations of analytes, one or more injections of MTBE should be made to ensure that accurate values are
obtained for the next sample.
6.6 Matrix interferences may be caused by contaminants that are coextracted from the sample. Also, note that all analytes listed
in Table 1 are not resolved from each other on any one column, that is, one analyte of interest may be an interferent for another
analyte of interest. The extent of matrix interferences will vary considerably from source to source, depending upon the water
sampled. The procedures in Section 16 can be used to overcome many of these interferences. Positive identifications should be
confirmed. See 16.6, 16.7, and 16.8.
6.7 It is important that samples and working standards be contained in the same solvent. The solvent for working standards must
be the same as the final solvent used in sample preparation. If this is not the case, chromatographic comparability of standards to
sample may be affected.
7. Apparatus and Equipment
7.1 Sample Bottle—Borosilicate amber, 1-L volume with graduations, fitted with screw caps lined with TFE-fluorocarbon.
Protect samples from light. The container must be washed and dried as described in 6.1.1 before use to minimize contamination.
Cap liners are cut to fit from sheets and extracted with methanol overnight prior to use.
The boldface numbers in parentheses refer to the list of references at the end of this test method.
D5317 − 98 (2017)
7.2 Glassware.
7.2.1 Separatory funnel, 2000-mL, with TFE-fluorocarbon stopcocks, ground glass or TFE-fluorocarbon stoppers.
7.2.2 Tumbler bottle, 1.7-L with TFE-fluorocarbon lined screw cap. Cap liners are cut to fit from sheets and extracted with
methanol overnight prior to use.
7.2.3 Concentrator tube, Kuderna-Danish (K-D), 10 or 25-mL, graduated. Calibration must be checked at the volumes
employed in the procedure. Ground-glass stoppers are used to prevent evaporation of extracts.
7.2.4 Evaporative flask, K-D, 500-mL. Attach to concentrator tube with springs.
7.2.5 Snyder column, K-D, three ball macro.
7.2.6 Snyder column, K-D, two ball micro.
7.2.7 Flask, round bottom, 500-mL with 24/40 ground glass joint.
7.2.8 Vials, glass, 5 to 10-mL capacity with TFE-fluorocarbon
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.