ASTM F2528-06
(Test Method)Standard Test Methods for Enteral Feeding Devices with a Retention Balloon
Standard Test Methods for Enteral Feeding Devices with a Retention Balloon
ABSTRACT
These test methods cover the establishment of performance requirements for the utilization of a single-use, enteral feeding device with a retention balloon, used by medical professionals for providing a means of nutrition and/or administration of medication to patients by means of natural orifice (nasal, oral, transluminal) and or a surgically created stoma. The product is manufactured in various sizes and materials such as silicone, urethane, and various polymers (as well as combinations of these) and is provided nonsterile for sterilization and sterile for single use only. The following test methods are: Flow rate through feeding lumen test method which covers the determination of flow rates through the drainage lumen of the enteral feeding device with retention balloon, balloon burst volume test method which covers the determination of balloon integrity of enteral feeding devices with retention balloon, balloon volume maintenance test method which is applicable enteral feeding devices with retention balloon to test the integrity of the inflation system to maintain balloon volume, balloon concentricity test method which is applicable enteral feeding devices with retention balloon to test the concentricy of the balloon, balloon size and shaft size test method which evaluates the retention balloon shaft size, balloon integrity test method which evaluates the integrtity of the retention balloon of the enteral feeding device, and balloon integrity in simulated gastric fluid test method which assesses the ability of the retention balloon to withstand gastric acidity levels without rupture, therefore, maintaining its functional purpose of retention.
SCOPE
1.1 These test methods cover the establishment of performance requirements for the utilization of a single-use, enteral feeding device with a retention balloon, used by medical professionals for providing a means of nutrition and/or administration of medication to patients by means of natural orifice (nasal, oral, transluminal) and or a surgically created stoma. The product is manufactured in various sizes and materials such as silicone, urethane, and various polymers (as well as combinations of these) and is provided nonsterile for sterilization and sterile for single use only. Rationale for these test methods can be found in Appendix X1.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to use.
General Information
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2528 − 06
StandardTest Methods for
Enteral Feeding Devices with a Retention Balloon
This standard is issued under the fixed designation F2528; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope administration of medication, or both, to patients by means of
natural orifice (nasal, oral, transluminal) or a surgically created
1.1 These test methods cover the establishment of perfor-
stoma, or both, consisting of a drainage lumen and inflation
mance requirements for the utilization of a single-use, enteral
lumen (see Fig. 1). Common balloon inflation sizes are 5 cm ,
feeding device with a retention balloon, used by medical
3 3
15 cm , and 20 cm .
professionals for providing a means of nutrition and/or admin-
istration of medication to patients by means of natural orifice
3.1.4 French size (Fr)—a scale used for denoting the size of
(nasal, oral, transluminal) and or a surgically created stoma.
catheters and other tubular instruments. The French size value
The product is manufactured in various sizes and materials
is three times the outer diameter of the tube as measured in
such as silicone, urethane, and various polymers (as well as
millimetres. For example, a diameter of 18 Fr indicates a
combinations of these) and is provided nonsterile for steriliza-
diameter of 6 mm.
tion and sterile for single use only. Rationale for these test
3.1.5 inflation volume—volume of liquid used to inflate the
methods can be found in Appendix X1.
retention balloon of the enteral feeding device for proposed
1.2 This standard does not purport to address all of the
testing in this standard.
safety concerns, if any, associated with its use. It is the
3.1.6 rated volume—stated volume of inflation of the reten-
responsibility of the user of this standard to establish appro-
tion balloon of the enteral feeding device in the manufacturers
priate safety and health practices and to determine the
labeling and instructions for use.
applicability of regulatory limitations prior to use.
3.1.7 simulated gastric fluid—a solution consisting of hy-
2. Referenced Documents
drochloric acid, salt and pepsin with a pH of approximately
2.1 ASTM Standards: 1.2, per USP standard recipe.
F623 Performance Specification for Foley Catheter
3.1.8 sterility—the state of being free from viable micro-
2.2 Other Standard:
organisms.
Simulated Gastric Fluid, USP Official Compendia of Stan-
dards
4. Specimen Preparation
3. Terminology
4.1 All test specimens for test methods listed below shall
consist of the manufacturers’ new, finished, untested, unsteril-
3.1 Definitions:
ized product.At the minimum, statistically valid samples of the
3.1.1 balloon integrity (resistance to rupture)—volume of
smallest and the largest diameter of enteral feeding devices
liquid that corresponds with balloon failure, or bursting.
shall be tested.
3.1.2 distal—refers to the balloon end of the enteral feeding
device
5. Test Methods
3.1.3 enteral feeding device with retention balloon—a two-
way medical device intended to provide a means of nutrition or
PROCEDURE A: FLOW RATE THROUGH FEEDING
LUMEN
These test methods are under the jurisdiction of ASTM Committee F04 on
5.1 Scope—This test method covers the determination of
Medical and Surgical Materials and Devices and is the direct responsibility of
flow rates through the drainage lumen of the enteral feeding
Subcommittee F04.35 on GI Appliations.
Current edition approved June 1, 2006. Published June 2006. DOI: 10.1520/ device with retention balloon.
F2528-06.
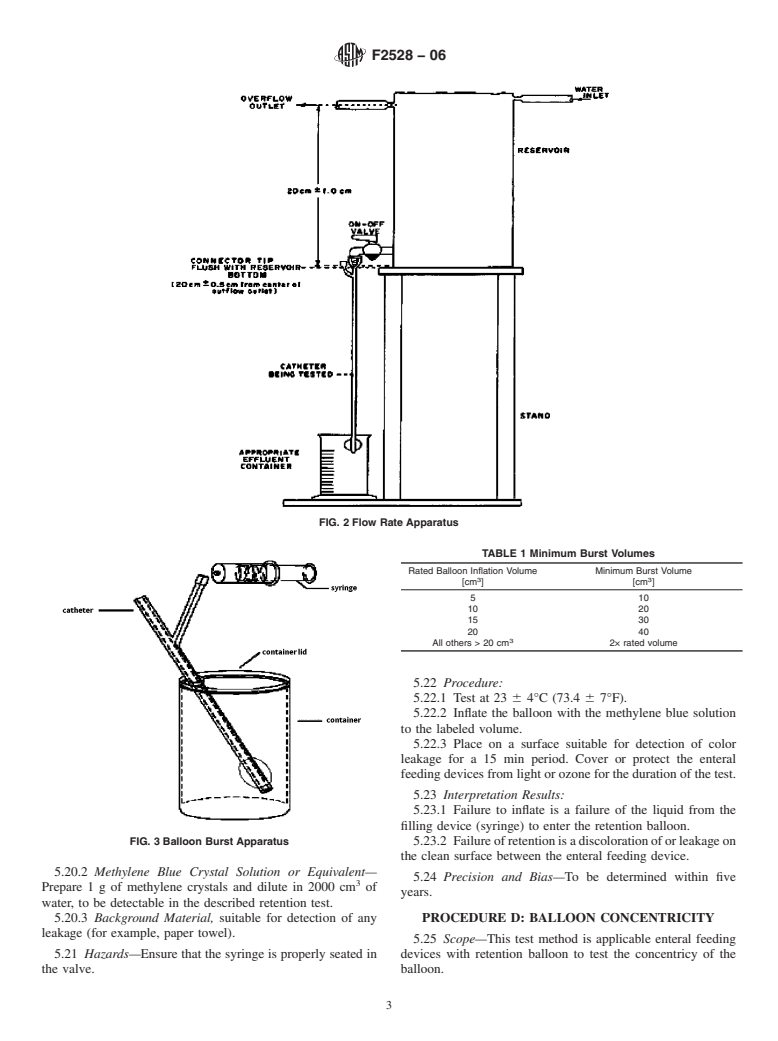
5.2 Summary of Test Method—The apparatus is set up as
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
shown in Fig. 2. The flow rate is adjusted through the water
Standards volume information, refer to the standard’s Document Summary page on
inlet to a rate sufficient to maintain flow through the overflow
the ASTM website.
3 outlet while each enteral feeding device is tested. A head
USP Official Compendia of Standards, available from U.S. Pharmacopeia
(USP), 12601 Twinbrook Pkwy., Rockville, MD 20852. pressure of 20 6 1.0 cm of water (196 6 10 kPa) above the
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2528 − 06
with water. The balloon is then inflated with water until
rupture, which enables the volume at which the balloon bursts
to be observed.
5.11 Significance and Use—The balloon burst volume is
measured to quantify the resistance of rupture of the enteral
feeding device with retention balloon member.
5.12 Apparatus—The testing apparatus is set up as shown in
Fig. 3.
FIG. 1 Enteral Feeding Device with Retention Balloon
5.12.1 System Reservoir.
5.12.2 Syringe.
tank bottom shall be maintained throughout the test to approxi-
5.12.3 Water.
mate actual physiological conditions. The overflow outlet
should not be covered by water.
5.13 Hazards—Water should be emptied from system res-
ervoir through purge valve when fill marked is reached.
5.3 Significance and Use—The flow rate is measured in
reverse flow for ease in testing, since differences in the flow
5.14 Procedure:
rate as a result of flow direction are theoretically insignificant.
5.14.1 Test at 23 6 4°C (73.4 6 7°F).
5.4 Apparatus:
5.14.2 Insert uninflated enteral feeding device into test
5.4.1 Water Reservoir, capable of maintaining 20 6 1.0 cm
orifice in system reservoir per Fig. 3.
(7.9 6 0.4 in.) of water (196 6 10 kPa) above the tip of the
5.14.3 Close orifice so that it is positioned proximal to the
enteral feeding device connection throughout the test as shown
enteral feeding device with retention balloon member. The
in Fig. 2. (See Performance Specification F623.)
device is not to be immersed in water within the reservoir per
5.4.2 Graduated Cylinder, calibrated for suitable measure-
Fig. 3.
ment of the effluent.
5.14.4 Fill syringe with amount of water greater than that
5.4.3 Syringe, with appropriate tip for inflation of enteral
listed in Table 1 for the desired French size. Attach tip of
feeding device balloon.
syringe to enteral feeding device inflation valve.
5.5 Hazards:
5.14.5 Inflate retention balloon at 1 cm /sec with water until
5.5.1 Overflow should not be covered. Head pressure must balloon bursts. Record amount of water injected into balloon at
be kept constant; water should always be exiting through the
time of burst.
overflow outlet.
5.15 Interpretation of Results—Burst volumes for enteral
5.5.2 Establish equilibrium before testing.
feeding devices tested must meet or exceed those listed in
5.5.3 Flow rates through all fittings must exceed that of the
Table 1.
enteral feeding device being tested.
5.16 Precision and Bias—To be determined within five
5.6 Procedure:
years.
5.6.1 Test at 23 6 4°C (73.4 6 7°F).
5.6.2 Inflate the retention balloon of the test specimen with
PROCEDURE C: BALLOON VOLUME
water to labeled volume.
MAINTENANCE
5.6.3 Connect the enteral feeding device to enteral feeding
5.17 Scope—This test method is applicable enteral feeding
device connector and open the stopcock. The tip of the enteral
devices with retention balloon to test the integrity of the
feeding device connection at the junction of enteral feeding
inflation system to maintain balloon volume.
device on-off valve should be level with the bottom of the tank
61 cm and it should deliver fluid at 20 6 1 cm (196 6 10 kPa)
5.18 Summary of Test Method—The balloon retention de-
head pressure at that junction.
vice of the enteral feeding device is inflated with a test liquid.
5.6.4 Establish flow equilibrium before taking test measure-
This test liquid contains a colorant which enables a leak of this
ments.
fluid to be observed. If no leak is observed, the integrity of the
5.6.5 Record the amount of fluid through the device feeding
inflation system is upheld, therefore maintaining the balloon
lumen in 30 seconds.
volume.
5.7 Interpretation of Results—Flow rates for enteral feeding
5.19 Significance and Use—This test method establishes a
devices tested must meet or exceed 9 cm /min.
standard test method for determining the functional integrity of
the inflation system of the enteral feeding device with retention
5.8 Precision and Bias—To be determined within five years.
balloon enteral feeding device by observing the consistancy of
PROCEDURE B: BALLOON BURST VOLUME
volume of the balloon after it is filled with test liquid.
Additionally, since it is the function of the inflated balloon to
5.9 Scope—This test method covers the determination of
retain the feeding device in position, the ballon must inflate,
balloon integrity of enteral feeding devices with retention
retain inflation volume, and release that volume when required.
balloon.
5.20 Apparatus:
5.10 Summary of Test Method—The enteral feeding device
with retention balloon is submerged in a small container filled 5.20.1 Syringe.
F2528 − 06
FIG. 2 Flow Rate Apparatus
TABLE 1 Minimum Burst Volumes
Rated Balloon Inflation Volume Minimum Burst Volume
3 3
[cm ] [cm ]
10 20
15 30
20 40
All others > 20 cm 2× rated volume
5.22 Procedure:
5.22.1 Test at 23 6 4°C (73.4 6 7°F).
5.22.2 Inflate the balloon with the methylene blue solution
to the labeled volume.
5.22.3 Place on a surface suitable for detection of color
leakage for a 15 min period. Cover or protect the enteral
feeding devices from light or ozone for the duration of the test.
5.23 Interpretation Results:
5.23.1 Failure to inflate is a failure of the liquid from the
filling device (syringe) to enter the retention balloon.
FIG. 3 Balloon Burst Apparatus 5.23.2 Failureofretentionisadiscolorationoforleakageon
the clean surface between the enteral feeding device.
5.20.2 Methylene Blue Crystal Solution or Equivalent—
5.24 Precision and Bias—To be determined within five
Prepare1gof methylene crystals and dilute in 2000 cm of
years.
water, to be detectable in the described retention test.
5.20.3 Background Material, suitable for detection of any PROCEDURE D: BALLOON CONCENTRICITY
leakage (for example, paper towel).
5.25 Scope—This test method is applicable enteral feeding
5.21 Hazards—Ensure that the syringe is properly seated in devices with retention balloon to test the concentricy of the
the valve. balloon.
F2528 − 06
TABLE 2 Concentricity Ratios
Rated Balloon Inflation Volume Maximum Concentricity
[cm ] Ratio
52:1
10 2:1
15 2:1
20 2:1
All others > 20 cm 2:1
5.36.1 French Size Calibration Gauge, tolerance of 60.13
FIG. 4 Concentricity Test Apparatus
mm (60.005 in.).
5.36.2 Metric Scale Rule.
5.37 Hazards:
5.26 Summary of Test Method—The retention balloon of the 5.37.1 No lubrication or undue force shall be applied to the
enteral feeding device is inflated with water, and with the use
enteral feeding device.
of a gauge, evaluted for concentricty. 5.37.2 The edges of each hole should be smooth to avoid
interference to the passage of the test enteral feeding device.
5.27 Significance and Use—This test is designed to quantify
balloon concentricity and the overall shape geometry of the 5.38 Procedure:
balloon. It is the purpose of the balloon to retain the feeding
5.38.1 Test at 23 6 4°C (73.4 6 7°F).
device in position during use, therefore, the balloon must be of
5.38.2 PerFig.5,withoutlubrication,pushtheproximalend
a functional uniformity that will not allow the enteral feeding of the uninflated enteral feeding device through the various
device to move from its desired position.
holes of the French size gauge, advancing it to the uninflated
balloon.
5.28 Apparatus—The testing apparatus is set up as shown in
5.38.3 Uninflated balloon should fit in appropriate French
Fig. 4.
3 3 3 size gauge hole snugly without undue insertion force. Label
5.28.1 Syringes—1cm,5cm , and 60 cm .
each test unit and the measured French size. Remove device
5.28.2 Water.
from gauge.
5.29 Hazards—Not applicable.
5.39 Interpretation of Results—The balloon section may
5.30 Procedure:
wrinkle but shall not tear or distort, and the enteral feeding
5.30.1 Test at 23 6 4°C (73.4 6 7°F).
device shaft or tip may offer resistance but if distortion or
5.30.2 Fill syringe with volume of water equal to balloon
stretching occurs it is considered a failure.
rating.
5.40 Precision and Bias—To be determined within five
5.30.3 Attach syringe to enteral feeding device inflation
years.
valve and inflate with water.
5.30.4 Per Fig. 4, use snap gauge and measure the two sides
PROCEDURE F: BALLOON INTEGRITY
of the balloon that visually appear to have the least symmetry.
5.41 Scope—This test method is to evaluate the integrtity of
Measurement should be taken 180° from each other.
the retention balloon of the enteral feeding device.
5.30.5 Divide larger measurement by smaller measurement
and quotient equals Concentricity Ratio. Tabulate all results.
5.42 Summary of Test Method—The retention balloons are
inflated with water and submerged in water at 37.8 6 3°C (100
5.31 Interpretation of Results—Balloon concentricity ratio
6 5°F) for seven days. The retention balloons are evaluated to
must not exceed those established in Table 2.
determine if they hold their integrity and do not rupture.
5.32 Precision and Bias—To be determined within five
5.43 Significance and Use—This test method is designed to
years.
subject the retention balloons to the inflation volume they
PROCEDURE E: BALLOON SIZE AND SHAFT SIZE
would be subjected to during use in the field, in order to
determine the integrity of the balloon. It is the purpose of the
5.33 Scope—This test method is to evaluate the retention
balloon to retain the feeding device in position during use,
balloon shaft size.
therefore, the performance of the retention balloon must be
5.34 Summary of Test Method—Using a French size gauge,
maintained and the balloon must not rupture.
the distal balloon tip is measured to determine the size of the
5.44 Apparatus—The testing apparatus is as shown in Fig.
retention balloon over the shaft.
6.
5.35 Significance and Use—The overall outside diameter of
5.44.1 Corrosion Resistant Tanks—The tanks should con-
the enteral feeding device during passage should conform to
tain no exposed iron, copper, or brass elements and have a
the required clinical orifice size.
removable mixing elements. The tanks should also have
5.36 Apparatus—The testing apparatus is as shown in Fig. sufficient covers/lids in order to prevent evaporation of solu-
5. tion.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.