ASTM F2077-00
(Test Method)Test Methods For Intervertebral Body Fusion Devices
Test Methods For Intervertebral Body Fusion Devices
SCOPE
1.1 This test method covers the materials and methods for the static and dynamic testing of intervertebral body fusion device assemblies, spinal implants designed to promote arthrodesis at a given spinal motion segment.
1.2 This test method is intended to provide a basis for the mechanical comparison among past, present, and future nonbiologic intervertebral body fusion device assemblies. This test method allows comparison of intervertebral body fusion device assemblies with different intended spinal locations and methods of application to the intradiscal spaces. This test method is intended to enable the user to compare intervertebral body fusion device assemblies mechanically and does not purport to provide performance standards for intervertebral body fusion device assemblies.
1.3 The test method describes static and dynamic tests by specifying load types and specific methods of applying these loads. These tests are designed to allow for the comparative evaluation of intervertebral body fusion device assemblies.
1.4 This test method does not purport to address expulsion testing of intervertebral body fusion device assemblies. However, since expulsion is a potential clinical failure mode, the user should address the implant's resistance to expulsion.
1.5 Guidelines are established for measuring displacements, determining the yield load or moment, evaluating the stiffness, and strength of the intervertebral body fusion device assemblies.
1.6 Some intervertebral body fusion device assemblies may not be testable in all test configurations.
1.7 The values stated in SI units are to be regarded as the standard with the exception of angular measurements, which may be reported in terms of either degrees or radians.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 2077 – 00
Test Methods For
Intervertebral Body Fusion Devices
This standard is issued under the fixed designation F 2077; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope E 4 Practices for Force Verification of Testing Machines
E 6 Terminology Relating to Methods of Mechanical Test-
1.1 This test method covers the materials and methods for
ing
the static and dynamic testing of intervertebral body fusion
E 1823 Terminology Relating to Fatigue and Fracture Test-
device assemblies, spinal implants designed to promote arthro-
ing
desis at a given spinal motion segment.
F 1582 Terminology Relating to Spinal Implants
1.2 This test method is intended to provide a basis for the
mechanical comparison among past, present, and future non-
3. Terminology
biologic intervertebral body fusion device assemblies. This test
3.1 For definition of terms refer to Terminology E 6,
method allows comparison of intervertebral body fusion device
E 1823, and F 1582.
assemblies with different intended spinal locations and meth-
3.2 Definitions of Terms Specific to This Standard:
ods of application to the intradiscal spaces. This test method is
3.2.1 coordinate system/axes, n—Three orthogonal axes are
intended to enable the user to mechanically compare interver-
defined by Terminology F 1582. The center of the coordinate
tebral body fusion device assemblies and does not purport to
system is located at the geometric center of the intervertebral
provide performance standards for intervertebral body fusion
body fusion device assembly. The XY plane is to bisect the
device assemblies.
sagittal plane angle between superior and inferior lines (sur-
1.3 The test method describes static and dynamic tests by
faces) that are intended to simulate the adjacent vertebral end
specifying load types and specific methods of applying these
plates. The positive Z axis is to be directed superiorly. Force
loads. These tests are designed to allow for the comparative
components parallel to the XY plane are shear components of
evaluation of intervertebral body fusion device assemblies.
loading. The compressive axial force is defined to be the
1.4 This test method does not purport to address expulsion
component in the negative Z direction. Torsional load is
testing of intervertebral body fusion device assemblies. How-
defined to be the component of moment parallel to the Z axis.
ever, since expulsion is a potential clinical failure mode, the
3.2.2 fatigue life, n—The number of cycles, N, that the
user should address the implant’s resistance to expulsion.
intervertebral body fusion device assembly can sustain at a
1.5 Guidelines are established for measuring displacements,
particular load or moment before mechanical or functional
determining the yield load or moment, evaluating the stiffness,
failure occurs.
and strength of the intervertebral body fusion device assem-
3.2.3 functional failure, n—Permanent deformation that
blies.
renders the intervertebral body fusion device assembly inef-
1.6 Some intervertebral body fusion device assemblies may
fective or unable to resist load and/or maintain attachment
not be testable in all test configurations.
adequately.
1.7 The values stated in SI units are to be regarded as the
3.2.4 ideal insertion location, n—The implant location with
standard with the exception of angular measurements, which
respect to the simulated inferior and superior vertebral bodies
may be reported in terms of either degrees or radians.
(polyacetal or metal blocks) dictated by the type, design, and
1.8 This standard does not purport to address all of the
manufacturer’s surgical installation instructions or the sur-
safety concerns, if any, associated with its use. It is the
geon’s preferred method of insertion.
responsibility of the user of this standard to establish appro-
3.2.5 intended method of application, n—Intervertebral
priate safety and health practices and determine the applica-
body fusion device assemblies may contain different types of
bility of regulatory limitations prior to use.
stabilizing anchors such as threads, spikes, and knurled sur-
2. Referenced Documents faces. Each type of anchor has an intended method of appli-
cation or attachment to the spine.
2.1 ASTM Standards:
3.2.6 intended spinal location, n—The anatomic region of
the spine intended for the intervertebral body fusion device
This test method is under the jurisdiction of ASTM Committee F04 on Medical
and Surgical Materials and Devices, and is the direct responsibility of Subcommittee
F04.25 on Spinal Devices.
Current edition approved December 2000. Published March 2001.
Annual Book of ASTM Standards, Vol 03.01.
Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
F 2077
assembly. Intervertebral body fusion device assemblies may be 3.2.16 test block, n—The component of the test apparatus
designed and developed for specific regions of the spine such for mounting the intervertebral body fusion device assembly
as the lumbar, thoracic, and cervical spine. Also, there exists for the intended test configuration.
different anatomical potential surgical approaches, which will 3.2.17 ultimate displacement (millimetres or degrees or
result in different implant orientation at different levels of the radians) (Displacement OF—Fig. 6), n—The displacement
spine. associated with the ultimate load or ultimate moment.
3.2.7 intervertebral body fusion device, n—A structure (bio- 3.2.18 ultimate load or moment (N or N*mm) (Point
logic or synthetic) that is placed in the disc space between two E—Fig. 6), n—The maximum applied load, F, transmitted by
adjacent vertebral bodies to provide support for eventual the pushrod (assumed equal to force component parallel to and
arthrodesis of the two adjacent vertebral bodies. indicated by load cell), or the applied moment about the Z axis
3.2.8 intradiscal height, n—The straight-line distance along that can be applied to an intervertebral body fusion device
the Z axis between the unaltered simulated vertebral bodies— assembly.
minimum height of 4 mm and a maximum height of 18 mm. 3.2.19 yield displacement (Distance OA—Fig. 6), n—The
See Fig. 1. displacement (mm) or angular displacement (deg) when an
3.2.9 load point, n—The point through which the resultant interbody fusion device asembly has a permanent deformation
force on the intervertebral device passes (that is, the geometric equal to the offset displacement or the offset angular displace-
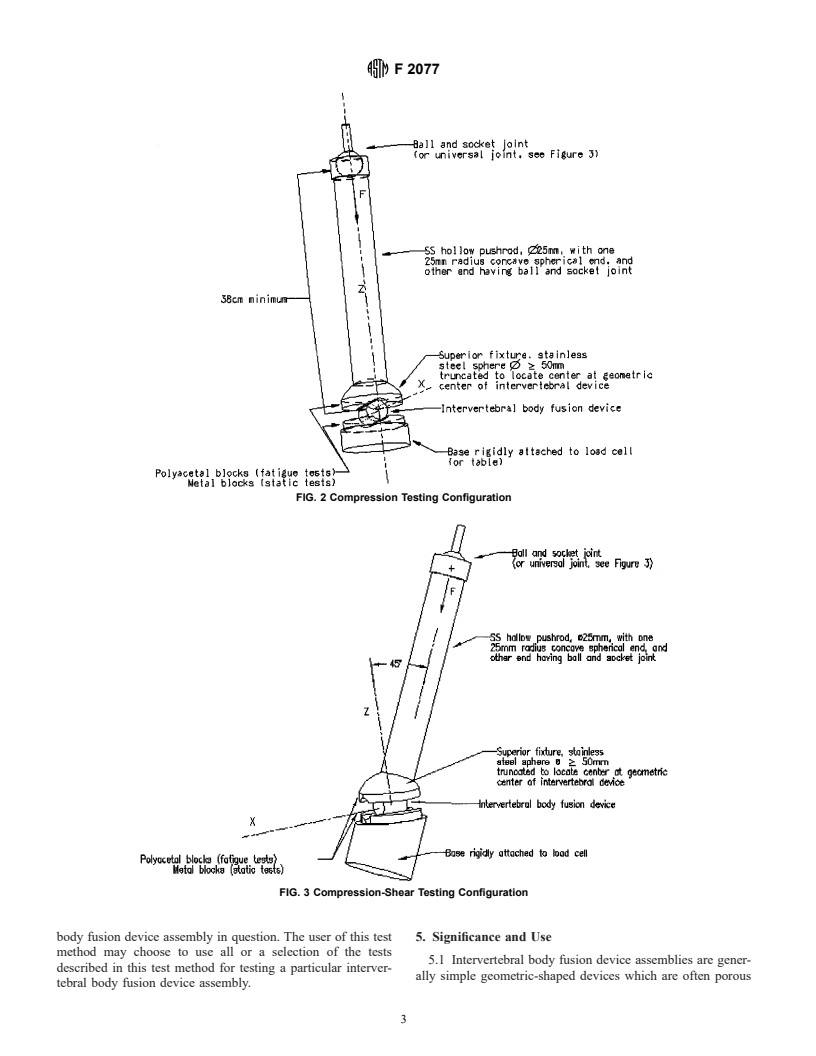
center of the superior fixture’s sphere) (Figs. 2-5). ment.
3.2.10 maximum run out load or moment, n—The maxi- 3.2.20 yield load or moment (Point D—Fig. 6), n—The
mum load or moment for a given test that can be applied to an applied load, F, transmitted by the pushrod (assumed equal to
intervertebral body fusion device assembly in which all of the force component parallel to and indicated by load cell), or the
tested constructs have withstood 5 000 000 cycles without applied moment about the Z axis required to produce a
functional or mechanical failure. permanent deformation equal to the offset displacement or the
3.2.11 mechanical failure, n—That associated with the on- offset angular displacement.
set of a defect in the material (that is, initiation of fatigue crack
4. Summary of Test Method
or surface wear).
3.2.12 offset angular displacement, n—(Distance OB—Fig. 4.1 These test methods are proposed for the mechanical
6)—Offset on the angular displacement axis equal to 10 % of testing of intervertebral body fusion device assemblies specific
the intradiscal height, H, divided by the outside diameter or to the lumbar, thoracic, and cervical spine.
height of the implant (maximum dimension of implant in XZ 4.2 Fatigue testing of the intervertebral body fusion device
plane if not cylindrical) (for example, for a 10-mm intradiscal assemblies will simulate a motion segment via a gap between
height and 16-mm intervertebral body fusion device assembly, two polyacetal test blocks. The polyacetal will eliminate the
distance OB = 10 mm/16 mm (0.10)(180°)/p = 3.6°). effects of the variability of bone properties and morphology for
3.2.13 offset displacement, n—(Distance OB—Fig. 6)— the fatigue tests. The minimum ultimate tensile strength of the
Offset on the displacement axis equal to 2 % of the intradiscal polyacetal blocks shall be no less than 61 MPa.
height (that is, 0.2 mm for a 10-mm intradiscal height). 4.3 Static testing of the intervertebral body fusion device
assemblies will simulate a motion segment via a gap between
3.2.14 permanent deformation, n—The remaining displace-
ment (mm or degrees or radians) relative to the initial unloaded two stainless steel blocks. The minimum ultimate tensile
strength of the blocks shall be no less than 1310 MPa.
condition of the intervertebral body fusion device assembly
after the applied load has been removed. 4.4 The pushrod shall also be manufactured from stainless
3.2.15 stiffness (N/mm or N*mm/Degree (Radian)) (The steel, which shall also have a minimum ultimate tensile
Slope of Line OG—Fig. 6), n—The slope of the initial linear strength no less than 1310 MPa.
portion of the load-displacement curve or the slope of the 4.5 Static and dynamic tests will evaluate the intervertebral
initial linear portion of the moment—angular displacement body fusion device assembly. The user of this test method must
curve. decide which series of tests are applicable to the intervertebral
FIG. 1 Intradiscal Height Diagram
F 2077
FIG. 2 Compression Testing Configuration
FIG. 3 Compression-Shear Testing Configuration
body fusion device assembly in question. The user of this test 5. Significance and Use
method may choose to use all or a selection of the tests
5.1 Intervertebral body fusion device assemblies are gener-
described in this test method for testing a particular interver-
ally simple geometric-shaped devices which are often porous
tebral body fusion device assembly.
F 2077
FIG. 4 Torsion Testing Configuration With Pin-Slot Gimbal
FIG. 5 Spherical Gimbal (Cross Section) for Torsion Testing Apparatus
or hollow in nature. Their function is to support the anterior body fusion device assemblies. These tests are conducted in
column of the spine to facilitate arthrodesis of the motion vitro to allow for analysis and comparison of the mechanical
segment. This test method outlines materials and methods for performance of intervertebral body fusion device assemblies to
the characterization and evaluation of the mechanical perfor- specific load modalities.
mance of different intervertebral body fusion device assemblies 5.3 The loads applied to the intervertebral body fusion
so that comparisons can be made between different designs. assemblies may differ from the complex loading seen in vivo,
5.2 This standard is designed to quantify the static and and therefore, the results from these tests may not directly
dynamic characteristics of different designs of intervertebral predict in vivo performance. The results, however, can be used
F 2077
FIG. 6 Typical Load Displacement Curve
to compare mechanical performance of different intervertebral material, or environment), all others must be kept constant to
body fusion device assemblies. facilitate interpretation of the results.
5.4 Since the environment may affect the dynamic perfor-
6. Apparatus
mance of intervertebral body fusion device assemblies, dy-
namic testing in a saline environment may be considered. 6.1 Test machines will conform to the requirements of
Fatigue tests should first be conducted in air (at ambient Practices E 4.
temperature) for comparison purposes since the environmental 6.2 The intradiscal height, H, shall be determined from
effects could be significant. If a simulated in vivo environment vertebral body and disc morphometric data at the intended
is desired, the investigator should consider testing in a saline level of application. Suggested heights are as follows: 10 mm
environmental bath at 37°C (for example, 0.9-g NaCl per for the lumbar spine, 6 mm for the thoracic spine, and 4 mm for
100-mL water) at a rate of 1 Hz or less. A simulated body fluid, the cervical spine. The intradiscal height should not reach zero
a saline drip or mist, distilled water, or other type of lubrication before the onset of functional or mechanical failure. If this
at 37°C could also be used with adequate justification. occurs, the test is considered a failure. The user of the test
5.5 If the devices are known to be temperature and envi- method should select the intradiscal height that is appropriate
ronment dependent, testing should be conducted in physiologic for the device being tested.
solution as described in 5.4. Devices that require physiologic 6.3 Axial Compression Test Apparatus
solution for testing should be tested in the same type solution The actuator of the testing machine is connected to the pushrod
for comparison purposes. by a minimal friction ball and socket joint or universal joint
5.6 The location within the simulated vertebral bodies and (that is, unconstrained in bending). The pushrod is connected to
position of the intervertebral body fusion device assembly with the superior fixture by a minimal friction sphere joint (that is,
respect to the loading axis will be dependent upon the design, unconstrained in bending and torsion). The hollow pushrod
the manufacturer’s recommendation, or the surgeon’s preferred should be of minimal weight so as to be considered a
method for implant placement. “two-force” member. It thus applies to the intervertebral body
5.7 It is well known that the failure of materials is depen- fusion device assembly a resultant force directed along the
dent upon stress, test frequency, surface treatments, and envi- pushrod’s axes and located at the center of the superior
ronmental factors. Therefore, when determining the effect of fixture’s sphere joint (the geometric center of the device being
changing one of these parameters (for example, frequency, tested). For the fatigue tests, the device is placed between two
F 2077
polyacetal blocks, which are rigidly attached to the metal assembly shall be previously unused parts only; no implants
shall be retested.
blocks (Fig. 2). For the static tests, metal blocks are to
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.