ASTM D5160-95(2008)
(Guide)Standard Guide for Gas-Phase Adsorption Testing of Activated Carbon
Standard Guide for Gas-Phase Adsorption Testing of Activated Carbon
SIGNIFICANCE AND USE
Activated carbon is used extensively for removing gases and vapors from air or other gas streams. The physical and chemical characteristics of an activated carbon can strongly influence its suitability for a given application. The procedure in this guide allows the evaluation of the dynamic adsorption characteristics of an activated carbon for a particular adsorbate under conditions chosen by the user. It is necessary that the user choose test conditions that are meaningful for the application (see Section 9).
This guide can also be used to evaluate activated carbons that have been impregnated with materials to enhance their effectiveness at removing gases otherwise poorly adsorbed on activated carbon.
The procedure given in this guide is not generally applicable for evaluation of carbons used as catalysts for such purposes as decomposition of low levels of ozone or oxidation of SO2 to SO 3.
The procedure given in this guide can be applied to reactivated or regenerated activated carbons.
Fig. 1 shows the adsorbate concentration profile in an activated carbon bed at breakthrough. The bed has a zone at the inlet in which the adsorbate concentration is equal to the influent concentration. In this region the carbon is at equilibrium with adsorbate. The adsorbate concentration in the remainder of the bed drops until at the outlet it is equal to the breakthrough concentration. The shorter the length of this mass transfer zone (adsorption zone), the more effectively the carbon in the bed is utilized. A bed whose depth is less than the length of this zone will show immediate appearance of adsorbate in the effluent (breakpoint).
From the standpoint of best carbon utilization it is desirable to choose a carbon which will give as short a mass transfer zone as possible under use conditions. However, in many applications, high adsorptive capacity is more important than a short mass transfer zone. In almost every application, bed pressure drop is also a primary consider...
SCOPE
1.1 This guide covers the evaluation of activated carbons for gas-phase adsorption. It presents a procedure for determining the dynamic adsorption capacity, No, and critical bed depth, d c, for an activated carbon used to remove a specific adsorbate from a gas stream under conditions chosen by the user.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific hazards statements are given in Section 8.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D5160 −95(Reapproved 2008)
Standard Guide for
Gas-Phase Adsorption Testing of Activated Carbon
This standard is issued under the fixed designation D5160; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 4. Summary of Guide
1.1 Thisguidecoverstheevaluationofactivatedcarbonsfor 4.1 An activated carbon bed that contains a known amount
gas-phase adsorption. It presents a procedure for determining ofcarbonischallengedwithanadsorbateinagasstreamunder
the dynamic adsorption capacity, N , and critical bed depth, d
conditions of flow rate, adsorbate concentration, temperature,
o
c, for an activated carbon used to remove a specific adsorbate pressure, and relative humidity set by the user. The time to
from a gas stream under conditions chosen by the user.
breakthrough of a specified concentration of adsorbate is
measured. The measurement is repeated using the same con-
1.2 The values stated in SI units are to be regarded as
ditions but varying the amount of carbon in the bed. For many
standard. No other units of measurement are included in this
practicalsystems,aplotofbreakthroughtimeversusamountof
standard.
carbon is linear. The slope and x-intercept of this line can be
1.3 This standard does not purport to address all of the
usedtocalculatethedynamiccapacity, N (expressedasgrams
o
safety concerns, if any, associated with its use. It is the 3
adsorbate/grams carbon or grams adsorbate/cm carbon) and
responsibility of the user of this standard to establish appro-
critical bed depth, d , characteristic of the activated carbon
c
priate safety and health practices and determine the applica-
under the conditions used in the test.
bility of regulatory limitations prior to use. Specific hazards
statements are given in Section 8.
5. Significance and Use
5.1 Activatedcarbonisusedextensivelyforremovinggases
2. Referenced Documents
and vapors from air or other gas streams. The physical and
2.1 ASTM Standards:
chemical characteristics of an activated carbon can strongly
D2652Terminology Relating to Activated Carbon
influence its suitability for a given application. The procedure
D2854Test Method for Apparent Density of Activated
in this guide allows the evaluation of the dynamic adsorption
Carbon
characteristics of an activated carbon for a particular adsorbate
D2867Test Methods for Moisture in Activated Carbon
under conditions chosen by the user. It is necessary that the
D3467Test Method for Carbon Tetrachloride Activity of
user choose test conditions that are meaningful for the appli-
Activated Carbon
cation (see Section 9).
E300Practice for Sampling Industrial Chemicals
5.2 This guide can also be used to evaluate activated
carbons that have been impregnated with materials to enhance
3. Terminology
their effectiveness at removing gases otherwise poorly ad-
3.1 Definitions:
sorbed on activated carbon.
3.1.1 breakthrough—the appearance in the effluent of a
5.3 The procedure given in this guide is not generally
specified concentration of an adsorbate of interest.
applicable for evaluation of carbons used as catalysts for such
3.1.2 Other terms relating to this guide are defined in
purposes as decomposition of low levels of ozone or oxidation
Terminology D2652.
of SO to SO .
2 3
5.4 The procedure given in this guide can be applied to
reactivated or regenerated activated carbons.
This guide is under the jurisdiction of ASTM Committee D28 on Activated
Carbon and is the direct responsibility of Subcommittee D28.04 on Gas Phase
5.5 Fig. 1 shows the adsorbate concentration profile in an
Evaluation Tests.
activatedcarbonbedatbreakthrough.Thebedhasazoneatthe
Current edition approved Aug. 1, 2008. Published September 2008. Originally
inlet in which the adsorbate concentration is equal to the
approved in 1991. Last previous edition approved in 2003 as D5160–95 (2003).
DOI: 10.1520/D5160-95R08.
influent concentration. In this region the carbon is at equilib-
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
rium with adsorbate. The adsorbate concentration in the
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
remainder of the bed drops until at the outlet it is equal to the
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. breakthroughconcentration.Theshorterthelengthofthismass
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5160−95 (2008)
diameter of the largest carbon particles present or 16 times the
mean diameter. The lower end of the tube must have a flat
support for the carbon bed. Care should be taken to ensure
uniformity of flow profile across the bed. The support should
contribute as little as possible to the total pressure drop of the
bed. For this reason, fritted glass supports are often undesir-
able. Fine mesh stainless steel screens supported if necessary
by heavier screens may be used. Commercially available
spunbonded polyester nonwovens having both high strength
and very low pressure drop may also be used as very
convenient supports for tests in small tubes.
NOTE 1—Atest fixture in which the bed is held in place at both top and
bottom requires less skill to obtain reproducible results. An 8.8 cm
diameter aluminum fixture with a perforated plate that screws down onto
thebedfromabovehasbeenusedsuccessfullyatbeddepthsfrom1to3.5
cm. A diagram of this fixture is shown in Fig. 2.
6.1.1 Flow should be downward through the sample to
FIG. 1 Concentration Profile of an Activated Carbon Bed at
avoiddisturbingthebed.Fortestsonsmallamountsofcarbon,
Breakthrough
a ground glass outer joint at the top of the tube allows easy
connection and disconnection from the challenge gas without
transferzone(adsorptionzone),themoreeffectivelythecarbon
disturbing the bed. It is very easy to disturb the packing of a
in the bed is utilized.Abed whose depth is less than the length
small bed. Preferably these should not be moved after loading.
of this zone will show immediate appearance of adsorbate in
6.1.2 The length of the sample tube must be several times
the effluent (breakpoint).
greaterthanthecriticalbeddepthoftheactivatedcarbonunder
5.6 From the standpoint of best carbon utilization it is
the test conditions chosen.
desirable to choose a carbon which will give as short a mass
6.2 Fill Device—For small beds the sample tube can be
transfer zone as possible under use conditions. However, in
loaded using the vibration feed device described in Test
many applications, high adsorptive capacity is more important
MethodD2854.Thebottomofthedeliveryfunnelshouldhave
than a short mass transfer zone. In almost every application,
the same diameter as the sample tube. It is desirable to allow
bed pressure drop is also a primary consideration.
thecarbontofallatleast10cmfromthebottomofthedelivery
5.7 Inafewsituationssuchasrespiratoryprotectionagainst
funnel to the top of the bed. For larger beds, the best packing
low levels of extremely toxic gases such as radioactive methyl
is obtained when the carbon falls through a loading column
iodide, a short mass transfer zone (that is, high adsorption rate
which contains screens to evenly distribute the carbon across
coefficient) is more important than ultimate capacity. In other
thebed. Thecolumnshouldhavethesamecrosssectionasthe
cases such as solvent recovery, a high dynamic capacity is
bed.
more important.
5.8 Although the design of adsorber beds is beyond the
British patent 606,867.
scope of this guide, the following points should be considered.
The bed diameter should be as large as possible in order to
lower the pressure drop and to maximize the amount of carbon
in the bed. Subject to pressure drop constraints, the deepest
possible carbon bed should be used. All else being equal, the
use of smaller particle size carbon will shorten the mass
transfer zone and improve bed efficiency at the expense of
higher pressure drop. If pressure drop considerations are
critical, some particle morphologies offer less resistance to
flow than others.
5.9 The two parameters obtained by the procedure in this
guidecanbeusedasanaidinselectinganactivatedcarbonand
in sizing the adsorption bed in which this carbon will be used.
The best carbon for most applications should have a high
dynamic capacity for the adsorbate N coupled with a short
o
mass transfer zone (small d ) when evaluated under the
c
operating conditions anticipated for the adsorber.
6. Apparatus
6.1 Sample Tube—This is often a vertically supported cy-
FIG. 2 Test Fixture for Gas-Phase Adsorption Testing of Acti-
lindrical glass tube with diameter at least twelve times the vated Carbon
D5160−95 (2008)
7. Hazards
7.1 Carbons containing toxic or radioactive adsorbates
should be disposed of in accordance with applicable federal,
state, and local regulations.
7.2 Certain gases and vapors have very high heats of
reaction as they chemisorb on a carbon surface. At high
concentrations, enough heat can be liberated to cause ignition
of the carbon bed if oxygen is present. An example is
chemisorptionofhighconcentrationsofphosphineorarsineon
whetlerized carbon.
7.3 Another hazard is encountered when large quantities of
easily oxidizable substances such as hydrazines are adsorbed
on carbon from an inert gas stream. When these carbons are
exposed to air, they often ignite as oxidation rapidly takes
place.Thesamematerialsadsorbedinlowconcentrationsfrom
an air stream cause no problems since the oxidation occurs
slowly during the adsorption process.
7.4 Adsorption of high concentrations of strong oxidizers
such as ozone (formation of ozonides), fluorine, hydrogen
peroxide, or nitric acid vapors can result in ignition or
explosion of the carbon bed.
8. Selection and Preparation of Activated Carbon
8.1 A representative sample should be obtained and pre-
pared for testing in accordance with Practice E300.
8.2 The particle size distribution of the activated carbon
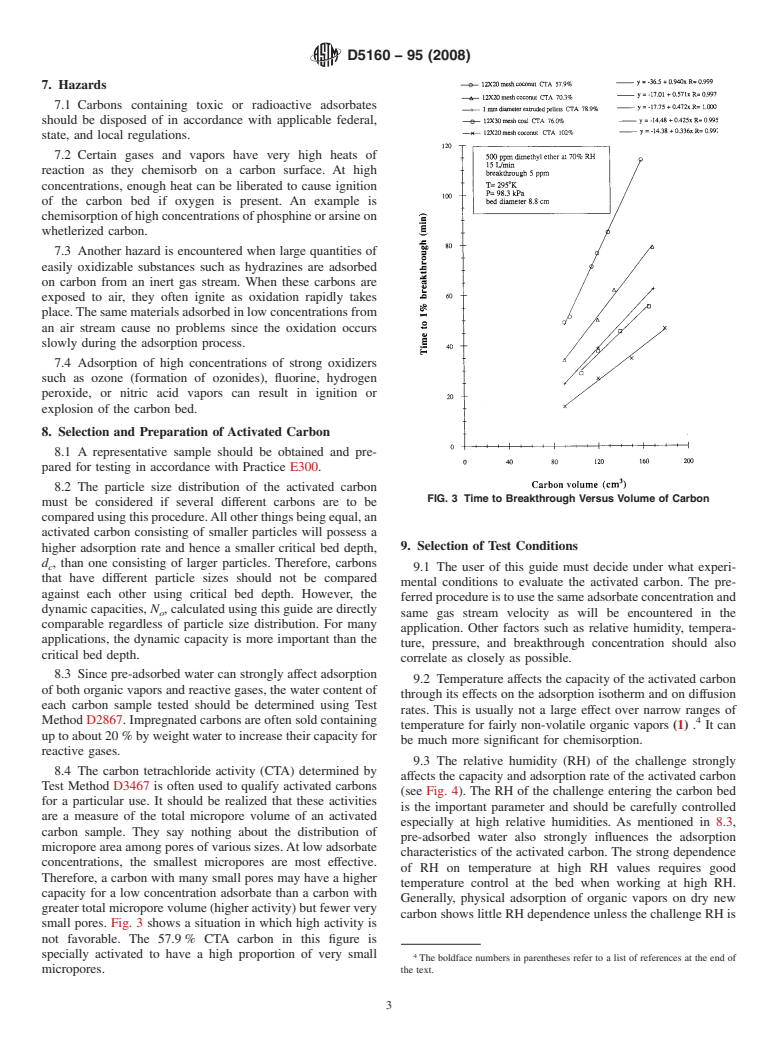
FIG. 3 Time to Breakthrough Versus Volume of Carbon
must be considered if several different carbons are to be
comparedusingthisprocedure.Allotherthingsbeingequal,an
activated carbon consisting of smaller particles will possess a
9. Selection of Test Conditions
higher adsorption rate and hence a smaller critical bed depth,
d , than one consisting of larger particles. Therefore, carbons
c 9.1 The user of this guide must decide under what experi-
that have different particle sizes should not be compared
mental conditions to evaluate the activated carbon. The pre-
against each other using critical bed depth. However, the
ferredprocedureistousethesameadsorbateconcentrationand
dynamic capacities, N , calculated using this guide are directly
o same gas stream velocity as will be encountered in the
comparable regardless of particle size distribution. For many
application. Other factors such as relative humidity, tempera-
applications, the dynamic capacity is more important than the
ture, pressure, and breakthrough concentration should also
critical bed depth.
correlate as closely as possible.
8.3 Since pre-adsorbed water can strongly affect adsorption
9.2 Temperature affects the capacity of the activated carbon
of both organic vapors and reactive gases, the water content of
through its effects on the adsorption isotherm and on diffusion
each carbon sample tested should be determined using Test
rates. This is usually not a large effect over narrow ranges of
MethodD2867.Impregnatedcarbonsareoftensoldcontaining
temperature for fairly non-volatile organic vapors (1) . It can
uptoabout20%byweightwatertoincreasetheircapacityfor
be much more significant for chemisorption.
reactive gases.
9.3 The relative humidity (RH) of the challenge strongly
8.4 The carbon tetrachloride activity (CTA) determined by
affects the capacity and adsorption rate of the activated carbon
Test Method D3467 is often used to qualify activated carbons
(see Fig. 4). The RH of the challenge entering the carbon bed
for a particular use. It should be realized that these activities
is the important parameter and should be carefully controlled
are a measure of the total micropore volume of an activated
especially at high relative humidities. As mentioned in 8.3,
carbon sample. They say nothing about the distribution of
pre-adsorbed water also strongly influences the adsorption
microporeareaamongporesofvarioussizes.Atlowadsorbate
characteristics of the activated carbon. The strong dependence
concentrations, the smallest micropores are most effective.
of RH on temperature at high RH values requires good
Therefore, a carbon with many small pores may have a higher
temperature control at the bed when working at high RH.
capacity for a low concentration adsorbate than a carbon with
Generally, physical adsorption of organic vapors on dry new
greatertotalmicroporevolume(higheractivity)butfewervery
carbon shows little RH dependence unless the challenge RH is
small pores. Fig. 3 shows a situation in which high activity is
not favorable. The 57.9% CTA carbon in this figure is
specially activated to have a high proportion of very small
The boldface numbers in parentheses refer to a list of references at the end of
micropores. the text.
D5160−95 (2008)
shown in Fig. 5. In this experiment, beds containing 105 cm
of carbon were tested against a 1000 ppm carbon tetrachloride
challenge at flow rates from 11 to 100 L/min. Breakthrough
was taken as 5 ppm. The data are plotted as time to break-
throughversusbedresidencetime.Bedresidencetimeisequal
tothebeddepthdividedbythesuperficialvelocity(volumetric
flow rate/cross section of the adsorbent bed) and can be
expressed in terms of the volume of adsorbent V (cm ) and the
flow rate Q (L/min) as follows:
V
τ s 50.06
~ ! S D
Q
The almost linear characteristic implies a dynamic adsorp-
tion capacity nearly independent of flow rate under these
conditions. In one case, dynamic capacity was found to be
invariant over a 30-fold range of flow rates (2).
10. Procedure
10.1 Generation of the Adsorbate in Carrier—A known
concentration of the adsorbate in a carrier gas must be
delivered to the carbon bed at a known delivery rate, a known
relative humidity, temperature, and pressure.
FIG. 4 Effect of Test Relative Humidity on the 1% Breakthrough
10.1.1 If the adsorbate is a liquid at room temperature, a
Time as a Function of Challenge Concentration
syringe pump can be used to deliver it into the metered gas
stream. For
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.