ASTM F2459-18
(Test Method)Standard Test Method for Extracting Residue from Metallic Medical Components and Quantifying via Gravimetric Analysis
Standard Test Method for Extracting Residue from Metallic Medical Components and Quantifying via Gravimetric Analysis
SIGNIFICANCE AND USE
5.1 This test method is suitable for determination of the total amount of extractable residue in metallic medical components. Extractable residue includes aqueous and non-aqueous residue, as well as non-soluble residue.
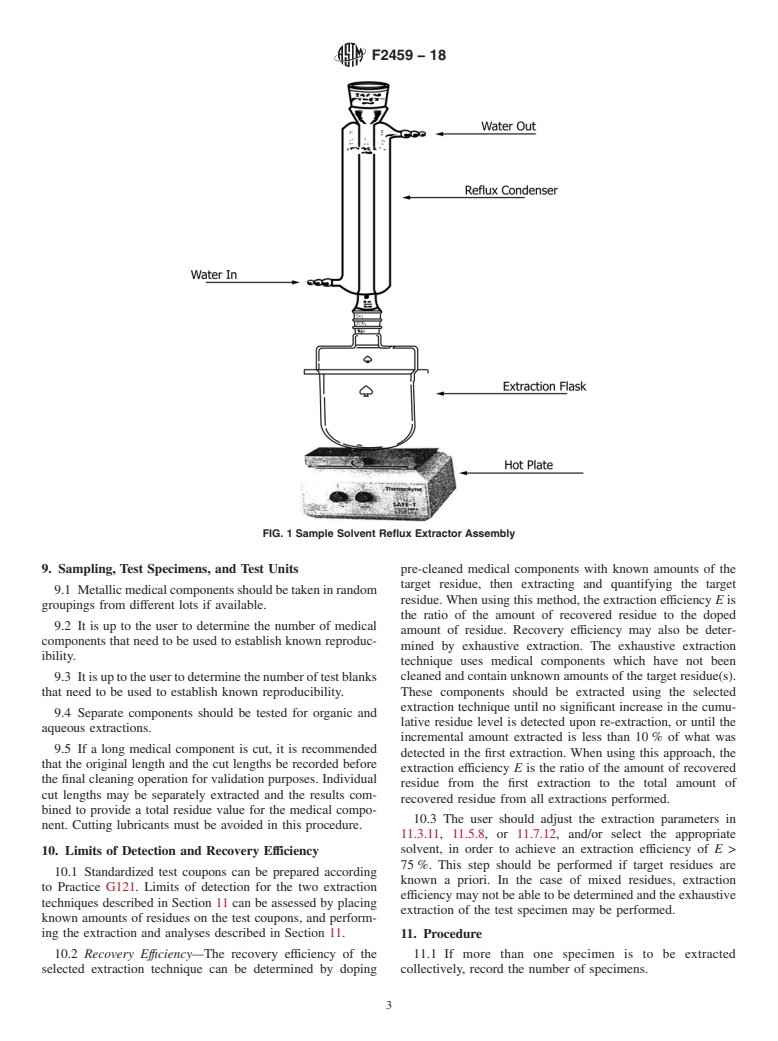
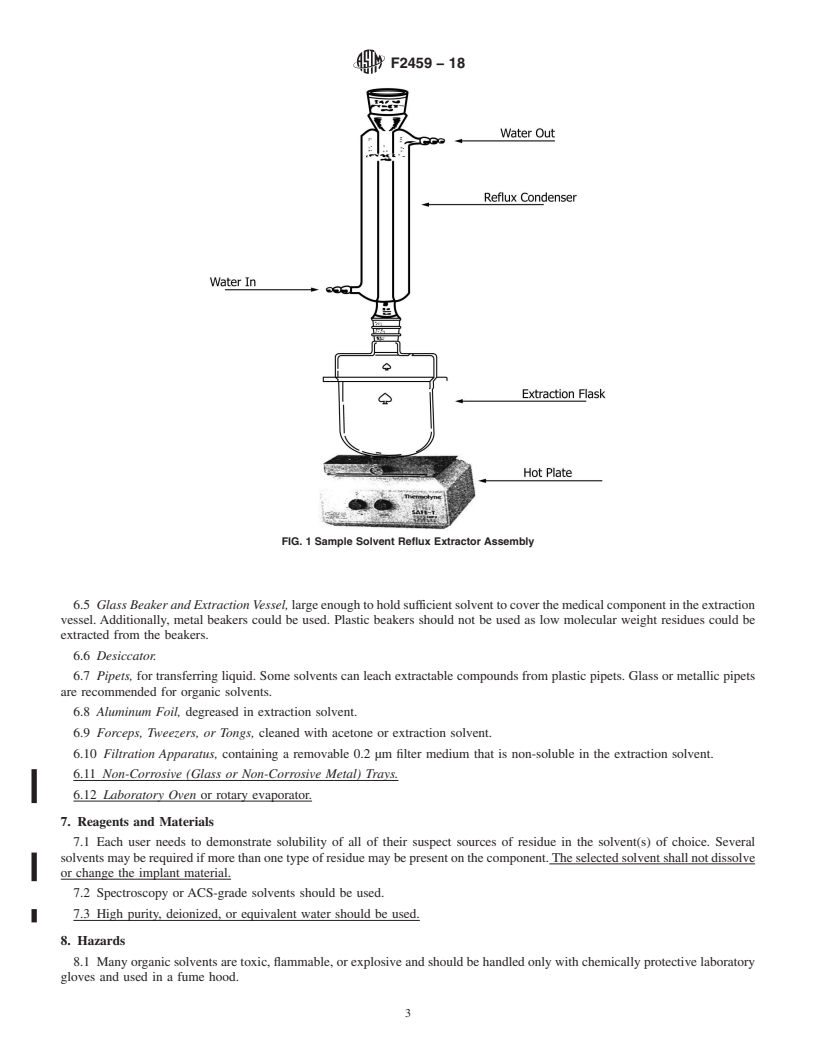
5.2 This test method recommends the use of a sonication technique to extract residue from the medical component. Other techniques, such as solvent reflux extraction, could be used but have been shown to be less efficient in some tests, as discussed in X1.2.
5.3 This test method is not applicable for evaluating the extractable residue for the reuse of a single-use component (SUD).
SCOPE
1.1 This test method covers the quantitative assessment of the amount of residue obtained from metallic medical components when extracted with aqueous or organic solvents.
1.2 This test method does not advocate an acceptable level of cleanliness. It identifies two techniques to quantify extractable residue on metallic medical components. In addition, it is recognized that this test method may not be the only method to determine and quantify extractables.
1.3 Although these methods may give the investigator a means to compare the relative levels of component cleanliness, it is recognized that some forms of component residue may not be accounted for by these methods.
1.4 The applicability of these general gravimetric methods have been demonstrated by many literature reports; however, the specific suitability for applications to all-metal medical components will be validated by an Interlaboratory Study (ILS) conducted according to Practice E691.
1.5 This test method is not intended to evaluate the residue level in medical components that have been cleaned for reuse. This test method is also not intended to extract residue for use in biocompatibility testing.
Note 1: For extraction of samples intended for the biological evaluation of devices or materials, refer to ISO 10993–12.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 This standard may involve hazardous or environmentally-restricted materials, operations, and equipment. This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.8 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2459 − 18
Standard Test Method for
Extracting Residue from Metallic Medical Components and
1
Quantifying via Gravimetric Analysis
This standard is issued under the fixed designation F2459; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.8 This international standard was developed in accor-
dance with internationally recognized principles on standard-
1.1 This test method covers the quantitative assessment of
ization established in the Decision on Principles for the
the amount of residue obtained from metallic medical compo-
Development of International Standards, Guides and Recom-
nents when extracted with aqueous or organic solvents.
mendations issued by the World Trade Organization Technical
1.2 This test method does not advocate an acceptable level
Barriers to Trade (TBT) Committee.
of cleanliness. It identifies two techniques to quantify extract-
able residue on metallic medical components. In addition, it is
2. Referenced Documents
recognized that this test method may not be the only method to
2
2.1 ASTM Standards:
determine and quantify extractables.
E691 Practice for Conducting an Interlaboratory Study to
1.3 Although these methods may give the investigator a
Determine the Precision of a Test Method
means to compare the relative levels of component cleanliness,
G121 Practice for Preparation of Contaminated Test Cou-
it is recognized that some forms of component residue may not
pons for the Evaluation of Cleaning Agents
be accounted for by these methods.
G131 Practice for Cleaning of Materials and Components by
1.4 The applicability of these general gravimetric methods Ultrasonic Techniques
G136 Practice for Determination of Soluble Residual Con-
have been demonstrated by many literature reports; however,
taminants in Materials by Ultrasonic Extraction
the specific suitability for applications to all-metal medical
3
componentswillbevalidatedbyanInterlaboratoryStudy(ILS)
2.2 ISO Standard:
conducted according to Practice E691.
ISO 10993–12 Biological Evaluation—Sample Preparation
and Reference Materials
1.5 This test method is not intended to evaluate the residue
level in medical components that have been cleaned for reuse.
3. Terminology
This test method is also not intended to extract residue for use
in biocompatibility testing.
3.1 Definitions:
3.1.1 ionic compounds/water soluble residue—residue that
NOTE 1—For extraction of samples intended for the biological evalu-
ation of devices or materials, refer to ISO 10993–12. is soluble in water, including surfactants and salts.
1.6 The values stated in SI units are to be regarded as 3.1.2 non-soluble debris—residue including metals, organic
standard. No other units of measurement are included in this solids, inorganic solids, and ceramics.
standard.
3.1.3 non-water soluble residue—residuesolubleinsolvents
1.7 This standard may involve hazardous or other than water. Inclusive in this are oils, greases,
environmentally-restricted materials, operations, and equip- hydrocarbons, and low molecular weight polymers. Typical
solvents used to dissolve these residues include chlorinated or
ment. This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the fluorinated solvents, or low molecular weight hydrocarbons.
responsibility of the user of this standard to establish appro-
3.1.4 reflux system—an apparatus containing an extraction
priate safety, health, and environmental practices and deter-
vessel and a solvent return system. It is designed to allow
mine the applicability of regulatory limitations prior to use.
1 2
This test method is under the jurisdiction ofASTM Committee F04 on Medical For referenced ASTM standards, visit the ASTM website, www.astm.org, or
and Surgical Materials and Devices and is the direct responsibility of Subcommittee contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
F04.15 on Material Test Methods. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Feb. 1, 2018. Published March 2018. Originally the ASTM website.
3
approved in 2005. Last previous edition approved in 2012 as F2459 – 12. DOI: Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
10.1520/F2459-18. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2459 − 12 F2459 − 18
Standard Test Method for
Extracting Residue from Metallic Medical Components and
1
Quantifying via Gravimetric Analysis
This standard is issued under the fixed designation F2459; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the quantitative assessment of the amount of residue obtained from metallic medical components
when extracted with aqueous or organic solvents.
1.2 This test method does not advocate an acceptable level of cleanliness. It identifies one techniquetwo techniques to quantify
extractable residue on metallic medical components. In addition, it is recognized that this test method may not be the only method
to determine and quantify extractables.
1.3 Although these methods may give the investigator a means to compare the relative levels of component cleanliness, it is
recognized that some forms of component residue may not be accounted for by these methods.
1.4 The applicability of these general gravimetric methods have been demonstrated by many literature reports; however, the
specific suitability for applications to all-metal medical components will be validated by an Interlaboratory Study (ILS) conducted
according to Practice E691.
1.5 This test method is not intended to evaluate the residue level in medical components that have been cleaned for reuse. This
test method is also not intended to extract residue for use in biocompatibility testing.
NOTE 1—For extraction of samples intended for the biological evaluation of devices or materials, refer to ISO 10993–12.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 This standard may involve hazardous or environmentally-restricted materials, operations, and equipment. This standard
does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this
standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the applicability of
regulatory limitations prior to use.
1.8 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
G121 Practice for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents
G131 Practice for Cleaning of Materials and Components by Ultrasonic Techniques
G136 Practice for Determination of Soluble Residual Contaminants in Materials by Ultrasonic Extraction
3
2.2 ISO Standard:
ISO 10993–12 Biological Evaluation—Sample Preparation and Reference Materials
3. Terminology
3.1 Definitions:
3.1.1 ionic compounds/water soluble residue—residue that is soluble in water, including surfactants and salts.
1
This test method is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.15 on Material Test Methods.
Current edition approved March 1, 2012Feb. 1, 2018. Published March 2012 March 2018. Originally approved in 2005. Last previous edition approved in 20052012 as
F2459 – 05.F2459 – 12. DOI: 10.1520/F2459-12.10.1520/F2459-18.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2459 − 18
3.1.2 non-soluble debris—residue including metals, organic solids, inorganic solids, and ceramics.
3.1.3 non-water soluble residue—residue soluble in solvents other than water. Inclusive in this are oils, greases,
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.