ASTM E2810-11(2017)
(Practice)Standard Practice for Demonstrating Capability to Comply with the Test for Uniformity of Dosage Units
Standard Practice for Demonstrating Capability to Comply with the Test for Uniformity of Dosage Units
SIGNIFICANCE AND USE
4.1 The methodology was originally developed (1-4)6 for use in drug content uniformity and dissolution but has general application to any multistage test with multiple acceptance criteria. Practice E2709 summarizes the statistical aspects of this methodology. This practice applies the general methodology of Practice E2709 specifically to the UDU test.
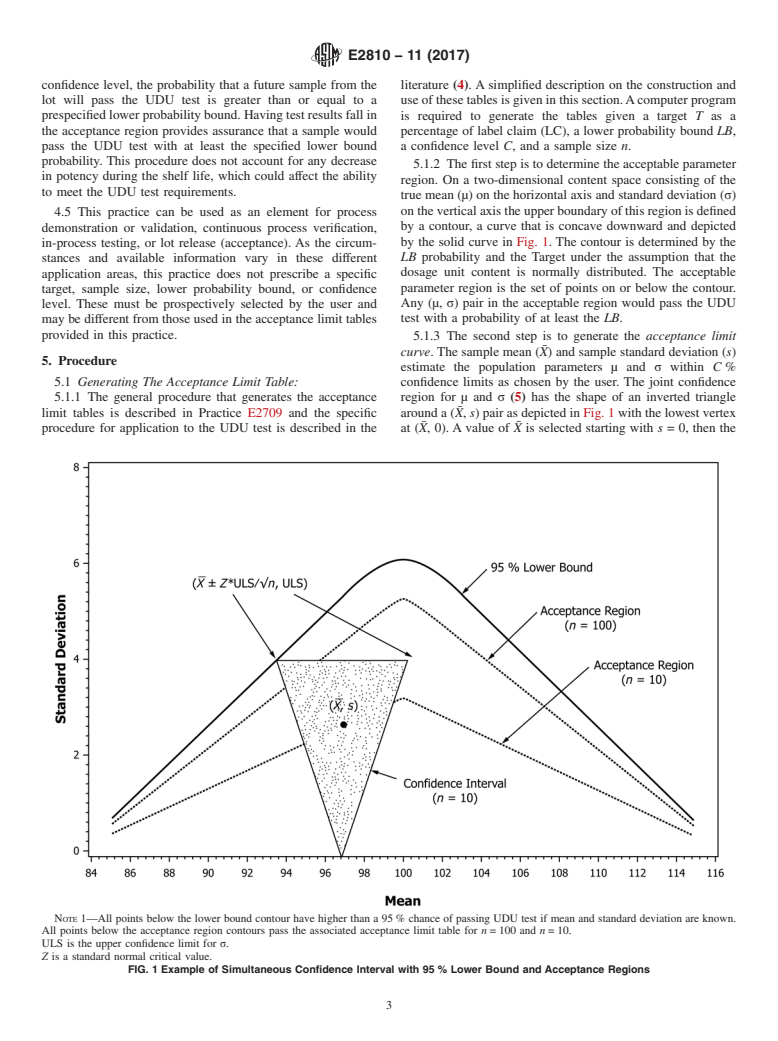
4.1.1 While other methods can be used to estimate the probability of passing the UDU test, they are outside the scope of this practice.
4.2 The UDU test procedure describes a two-stage sampling test, where at each stage one can pass or continue testing, and the decision to fail is deferred until the second stage. At each stage there are acceptance criteria on the test results as outlined in Table 1.
4.3 The UDU test is a market standard. The USP General Notices include the following statement about compendial standards. “The similarity to statistical procedures may seem to suggest an intent to make inference to some larger group of units, but in all cases, statements about whether the compendial standard is met apply only to the units tested.” Therefore, the UDU procedure is not intended for inspecting uniformity of finished product for lot/batch release or as a lot inspection procedure.
4.3.1 The UDU test defines a product requirement to be met at release and throughout the shelf-life of the product.
4.3.2 Passing the UDU test once does not provide statistical assurance that a batch of drug product meets specified statistical quality control criteria.
4.4 This practice provides a practical specification that may be applied when uniformity of dosage units is required. An acceptance region for the mean and standard deviation of a set of test results from the lot is defined such that, at a prescribed confidence level, the probability that a future sample from the lot will pass the UDU test is greater than or equal to a prespecified lower probability bound. Having test results fall in the acceptance re...
SCOPE
1.1 This practice provides a general procedure for evaluating the capability to comply with the Uniformity of Dosage Units (UDU) test. This test is given in General Chapter Uniformity of Dosage Units of the USP, in 2.9.40 Uniformity of Dosage Units of the Ph. Eur., and in 6.02 Uniformity of Dosage Units of the JP, and these versions are virtually interchangeable. For this multiple-stage test, the procedure computes a lower bound on the probability of passing the UDU test, based on statistical estimates made at a prescribed confidence level from a sample of dosage units.
1.2 This methodology can be used to generate an acceptance limit table, which defines a set of sample means and standard deviations that assures passing the UDU test for a prescribed lower probability bound, confidence level, and sample size.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2810 − 11 (Reapproved 2017)
Standard Practice for
Demonstrating Capability to Comply with the Test for
Uniformity of Dosage Units
This standard is issued under the fixed designation E2810; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E2709 Practice for Demonstrating Capability to Comply
with an Acceptance Procedure
1.1 This practice provides a general procedure for evaluat-
2.2 Other Documents:
ing the capability to comply with the Uniformity of Dosage
JP Japanese Pharmacopoeia
Units (UDU) test. This test is given in General Chapter <905>
Ph. Eur. European Pharmacopoeia
Uniformity of Dosage Units of the USP, in 2.9.40 Uniformity
USP United States Pharmacopeia
of Dosage Units of the Ph. Eur., and in 6.02 Uniformity of
Dosage Units of the JP, and these versions are virtually
3. Terminology
interchangeable. For this multiple-stage test, the procedure
3.1 Definitions—See Terminology E2363 for a more exten-
computesalowerboundontheprobabilityofpassingtheUDU
sive listing of terms in ASTM Committee E55 standards.
test, based on statistical estimates made at a prescribed
3.2 Definitions of Terms Specific to This Standard:
confidence level from a sample of dosage units.
3.2.1 acceptable parameter region, n—the set of values of
1.2 Thismethodologycanbeusedtogenerateanacceptance
parameters characterizing the distribution of test results for
limit table, which defines a set of sample means and standard
which the probability of passing the lot acceptance procedure
deviations that assures passing the UDU test for a prescribed
is greater than a prescribed lower bound.
lower probability bound, confidence level, and sample size.
3.2.2 acceptance limit, n—the boundary of the acceptance
1.3 This standard does not purport to address all of the
region, for example, the maximum sample standard deviation
safety concerns, if any, associated with its use. It is the
for a given sample mean.
responsibility of the user of this standard to establish appro-
3.2.2.1 Discussion—The coefficient of variation (relative
priate safety, health, and environmental practices and deter-
standard deviation) may be substituted for the standard devia-
mine the applicability of regulatory limitations prior to use.
tion where applicable.
1.4 This international standard was developed in accor-
3.2.3 acceptance region, n—the set of values of parameter
dance with internationally recognized principles on standard-
estimates (that is, sample mean and standard deviation) where
ization established in the Decision on Principles for the
confidence limits attain a prescribed lower bound on the
Development of International Standards, Guides and Recom-
probability of passing a lot acceptance procedure.
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee.
3.2.4 confidence level, C, n—the prescribed overall level for
calculating the uncertainty region of the parameters from the
2. Referenced Documents
sample estimates.
2.1 ASTM Standards:
3.2.4.1 Discussion—The preset confidence level is stated as
E2363 Terminology Relating to ProcessAnalytical Technol-
a percentage, for example, 100 (1 –α) = 95 %, where α is a
ogy in the Pharmaceutical Industry
risk that is allocated to the two parameters being estimated.
3.2.5 lowerprobabilitybound,LB,n—thenominalprobabil-
ity of passing the UDU test for a given set of parameter
estimates.
This practice is under the jurisdiction of ASTM Committee E55 on Manufac-
ture of Pharmaceutical and Biopharmaceutical Products and is the direct responsi-
bility of Subcommittee E55.03 on General Pharmaceutical Standards. Available from the Pharmaceuticals and Medical Devices Agency (PMDA),
Current edition approved Oct. 1, 2017. Published October 2017. Originally Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013,
ɛ2
approved in 2011. Last previous edition approved in 2011 as E2810 – 11 . DOI: Japan, https://www.pmda.go.jp.
10.1520/E2810-11R17. Available from the European Directorate for the Quality of Medicines and
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Health Care (EDQM), Council of Europe, 7 allée Kastner, CS 30026, F-67081
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Strasbourg, France, http://www.edqm.eu.
Standards volume information, refer to the standard’s Document Summary page on Available from U.S. Pharmacopeial Convention (USP), 12601 Twinbrook
the ASTM website. Pkwy., Rockville, MD 20852-1790, http://www.usp.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2810 − 11 (2017)
3.2.6 multiple-stage acceptance procedure, n—a procedure this methodology. This practice applies the general methodol-
that involves more than one stage of sampling and testing a ogy of Practice E2709 specifically to the UDU test.
given quality characteristic with one or more acceptance
4.1.1 While other methods can be used to estimate the
criteria per stage. probability of passing the UDU test, they are outside the scope
of this practice.
3.2.7 representative sample, n—a sample that consists of a
number of units that are drawn based on rational criteria such
4.2 TheUDUtestproceduredescribesatwo-stagesampling
as random sampling and intended to assure that the sample
test, where at each stage one can pass or continue testing, and
accurately portrays the material being sampled
the decision to fail is deferred until the second stage. At each
stagethereareacceptancecriteriaonthetestresultsasoutlined
3.2.8 sampling plan, n—scheme for selecting dosage units
in Table 1.
from locations within a batch for testing purposes.
3.2.8.1 Discussion—In this standard, a single dosage unit is
4.3 The UDU test is a market standard. The USP General
selected from each batch location.
Notices include the following statement about compendial
3.2.9 uniformity of dosage units, UDU, n—the degree of standards.“Thesimilaritytostatisticalproceduresmayseemto
uniformity in the amount of the drug substance among dosage suggest an intent to make inference to some larger group of
units. units,butinallcases,statementsaboutwhetherthecompendial
3.2.9.1 Discussion—The requirements of the UDU test ap- standard is met apply only to the units tested.” Therefore, the
ply to each drug substance in dosage units containing one or UDU procedure is not intended for inspecting uniformity of
more drug substances, unless otherwise specified. The unifor- finished product for lot/batch release or as a lot inspection
mity improves as the variability decreases. procedure.
4.3.1 The UDU test defines a product requirement to be met
4. Significance and Use
at release and throughout the shelf-life of the product.
4.1 The methodology was originally developed (1-4) for 4.3.2 Passing the UDU test once does not provide statistical
assurance that a batch of drug product meets specified statis-
use in drug content uniformity and dissolution but has general
application to any multistage test with multiple acceptance tical quality control criteria.
criteria. Practice E2709 summarizes the statistical aspects of
4.4 This practice provides a practical specification that may
be applied when uniformity of dosage units is required. An
acceptance region for the mean and standard deviation of a set
The boldface numbers in parentheses refer to a list of references at the end of
of test results from the lot is defined such that, at a prescribed
this standard.
TABLE 1 Uniformity of Dosage Units Test Procedure
NOTE 1—All measurements of dosage units and criteria values are in
percentagelabelclaim(%LC).Ateachstagecalculatethesampleaverage,
X—, and the sample standard deviation, s.
Number
Stage Pass Stage If:
Tested
¯
S 10 |M – X|+2.4 s ≤ 15.0, where M is
defined below.
¯
S 20 (1)|M – X|+2.0 s ≤ 15.0, using all
30 results (S +S ).
1 2
(2) No dosage unit is outside the
maximum allowed range of
0.75 * M to 1.25 * M.
M is defined as follows:
If T is less than or equal to 101.5 %LC, and
¯
(1)If X is less than 98.5 %LC, then
M = 98.5 %LC.
¯
(2)If X is between 98.5 and
¯
101.5 %LC, then M = X.
¯
(3)If X is greater than 101.5 %LC,
then M = 101.5 %LC.
If T is greater than 101.5 %LC, and
¯
(1)If X is less than 98.5 %LC, then
M = 98.5 %LC.
¯
(2)If X is between 98.5 and T, then
¯
M = X.
¯
(3)If X is greater than T, then
M = T.
T is the target content per dosage unit at the time of manufacture, ex-
pressed as %LC. Unless otherwise specified in the individual monograph, T
is 100.0 %LC.
E2810 − 11 (2017)
confidence level, the probability that a future sample from the literature (4). A simplified description on the construction and
lot will pass the UDU test is greater than or equal to a use of these tables is given in this section.Acomputer program
prespecifiedlowerprobabilitybound.Havingtestresultsfallin is required to generate the tables given a target T as a
the acceptance region provides assurance that a sample would percentage of label claim (LC), a lower probability bound LB,
pass the UDU test with at least the specified lower bound
a confidence level C, and a sample size n.
probability. This procedure does not account for any decrease
5.1.2 The first step is to determine the acceptable parameter
in potency during the shelf life, which could affect the ability
region. On a two-dimensional content space consisting of the
to meet the UDU test requirements.
true mean (µ) on the horizontal axis and standard deviation (σ)
ontheverticalaxistheupperboundaryofthisregionisdefined
4.5 This practice can be used as an element for process
by a contour, a curve that is concave downward and depicted
demonstration or validation, continuous process verification,
by the solid curve in Fig. 1. The contour is determined by the
in-process testing, or lot release (acceptance). As the circum-
LB probability and the Target under the assumption that the
stances and available information vary in these different
dosage unit content is normally distributed. The acceptable
application areas, this practice does not prescribe a specific
parameter region is the set of points on or below the contour.
target, sample size, lower probability bound, or confidence
Any (µ, σ) pair in the acceptable region would pass the UDU
level. These must be prospectively selected by the user and
may be different from those used in the acceptance limit tables test with a probability of at least the LB.
provided in this practice.
5.1.3 The second step is to generate the acceptance limit
¯
curve. The sample mean (X) and sample standard deviation (s)
5. Procedure
estimate the population parameters µ and σ within C %
5.1 Generating The Acceptance Limit Table:
confidence limits as chosen by the user. The joint confidence
5.1.1 The general procedure that generates the acceptance region for µ and σ (5) has the shape of an inverted triangle
¯
limit tables is described in Practice E2709 and the specific around a (X, s) pair as depicted in Fig. 1 with the lowest vertex
¯ ¯
procedure for application to the UDU test is described in the at (X, 0). A value of X is selected starting with s = 0, then the
NOTE 1—All points below the lower bound contour have higher than a 95 % chance of passing UDU test if mean and standard deviation are known.
All points below the acceptance region contours pass the associated acceptance limit table for n = 100 and n = 10.
ULS is the upper confidence limit for σ.
Z is a standard normal critical value.
FIG. 1 Example of Simultaneous Confidence Interval with 95 % Lower Bound and Acceptance Regions
E2810 − 11 (2017)
confidence region is expanded by increasing s until one of the 5.2 Using the Acceptance Limit Tables in This Practice:
upper vertices just touches the acceptable parameter region.
5.2.1 In each table acceptance limits on the standard devia-
The size of the confidence region is determined by C and n.
tion are given for means ranging 90–110 % of LC in incre-
This value of s defines a point on the acceptance limit curve at
ments of 0.2 %LC for sample sizes ranging from n=10 to
¯ ¯
(X, s).Additional selections of X then generate the acceptance
n = 500. In all tables the target is set at T = 100 %LC, so the
limit curve, as depicted as dotted lines in Fig. 1. Acceptance
acceptance limits for standard deviations are symmetrical
limit curves are shown for n = 10 and n = 100, illustrating that
around 100 %LC. This target is also required for interchange-
theacceptancelimitsapproachtheacceptableparameterregion
ability across the ICH regions (6).
with increasing sample size.
5.2.1.1 At the confidence level of C = 95 % used often in
5.1.4 Computer programs have been developed for generat-
the regulatory arena, three levels of the probability lower
ing acceptance limit tables, but these may not be available to
bound are provided: LB=90%(Table 2), LB=95%(Table 3)
all practitioners. This practice contains four acceptance limit
and LB=99%(Table 4).These provide 90 %, 95 %, and 99 %
tables for many practical use situations.
TABLE 2 Acceptance Limits on Sample Standard Deviation (%LC) for
T = 100 %LC,C =95%,LB =90%LC
Sample Average Sample Size (n)
(%LC) 10 30 40 50 60 80 100 120 150 200 500
100.0 2.91 4.36 4.65 4.84 4.99 5.19 5.33 5.43 5.54 5.66 5.93
99.8 or 100.2 2.88 4.31 4.59 4.79 4.94 5.14 5.28 5.38 5.50 5.62 5.91
99.6 or 100.4 2.84 4.26 4.54 4.74 4.89 5.09 5.24 5.34 5.45 5.58 5.88
99.4 or 100.6 2.81 4.21 4.49 4.69 4.83 5.04 5.18 5.29 5.40 5.53 5.84
99.2 or 100.8 2.77 4.16 4.43 4.63 4.77 4.98 5.13 5.23 5.35 5.48 5.79
99.0 or 101.0 2.74 4.10 4.38 4.57 4.72 4.92 5.07 5.17 5.29 5.43 5.74
98.8 or 101.2 2.70 4.05 4.32 4.52 4.66 4.86 5.01 5.11 5.23 5.37 5.69
98.6 or 101.4 2.67 4.00 4.27 4.46 4.60 4.80 4.94 5.05 5.17 5.30 5.63
98.4 or 101.6 2.63 3.95 4.21 4.40 4.54 4.74 4.88 4.99 5.10 5.24 5.56
98.2 or 101.8 2.60 3.89 4.16 4.34 4.48 4.68 4.82 4.92 5.04 5.17 5.49
98.0 or 102.0 2.56 3.84 4.10 4.28 4.42 4.62 4.75 4.86 4.97 5.10 5.43
97.8 or 102.2 2.53 3.79 4.05 4.22 4.36 4.55 4.69 4.79 4.90 5.03 5.35
97.6 or 102.4 2.49 3.74 3.99 4.17 4.30 4.49 4.62 4.72 4.84 4.97 5.28
97.4 or 102.6 2.46 3.68 3.93 4.11 4.24 4.43 4.56 4.66 4.77 4.90 5.21
97.2 or 102.8 2.42 3.63 3.88 4.05 4.18 4.36 4.50 4.59 4.70 4.83 5.13
97.0 or 103.0 2.39 3.58 3.82 3.99 4.12 4.30 4.43 4.53 4.63 4.76 5.06
96.8 or 103.2 2.35 3.53 3.77 3.93 4.06 4.24 4.37 4.46 4.56 4.69 4.99
96.6 or 103.4 2.32 3.48 3.71 3.87 4.00 4.18 4.30 4.39 4.50 4.62 4.91
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´2
Designation: E2810 − 11 E2810 − 11 (Reapproved 2017)
Standard Practice for
Demonstrating Capability to Comply with the Test for
Uniformity of Dosage Units
This standard is issued under the fixed designation E2810; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—Editorial corrections made throughout in February 2013.
ε NOTE—Editorial corrections made throughout in December 2013.
1. Scope
1.1 This practice provides a general procedure for evaluating the capability to comply with the Uniformity of Dosage Units
(UDU) test. This test is given in General Chapter <905> Uniformity of Dosage Units of the USP, in 2.9.40 Uniformity of Dosage
Units of the Ph. Eur., and in 6.02 Uniformity of Dosage Units of the JP, and these versions are virtually interchangeable. For this
multiple-stage test, the procedure computes a lower bound on the probability of passing the UDU test, based on statistical estimates
made at a prescribed confidence level from a sample of dosage units.
1.2 This methodology can be used to generate an acceptance limit table, which defines a set of sample means and standard
deviations that assures passing the UDU test for a prescribed lower probability bound, confidence level, and sample size.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
E2363 Terminology Relating to Process Analytical Technology in the Pharmaceutical Industry
This practice is under the jurisdiction of ASTM Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
Subcommittee E55.03 on General Pharmaceutical Standards.
Current edition approved Oct. 1, 2011Oct. 1, 2017. Published December 2011October 2017. Originally approved in 2011. Last previous edition approved in 2011 as E2810
ɛ2
– 11 . DOI: 10.1520/E2810-11E02.10.1520/E2810-11R17.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2810 − 11 (2017)
E2709 Practice for Demonstrating Capability to Comply with an Acceptance Procedure
2.2 Other Documents:
JP Japanese Pharmacopoeia
Ph. Eur. European Pharmacopoeia
USP United States Pharmacopeia
3. Terminology
3.1 Definitions—See Terminology E2363 for a more extensive listing of terms in ASTM Committee E55 standards.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 acceptable parameter region, n—the set of values of parameters characterizing the distribution of test results for which
the probability of passing the lot acceptance procedure is greater than a prescribed lower bound.
3.2.2 acceptance limit, n—the boundary of the acceptance region, for example, the maximum sample standard deviation for a
given sample mean.
Available from the Pharmaceuticals and Medical Devices Agency, Japan, http://jpdb.nihs.go.jp.Agency (PMDA), Shin-Kasumigaseki Building, 3-3-2 Kasumigaseki,
Chiyoda-ku, Tokyo 100-0013, Japan, https://www.pmda.go.jp.
Available from the European Council, Directorate for the Quality of Medicines and Health Care (EDQM), Council of Europe, 7 allée Kastner, CS 30026, F-67081
Strasbourg, France, http://www.edqm.eu.
Available from U.S. Pharmacopeia Pharmacopeial Convention (USP), 12601 Twinbrook Pkwy., Rockville, MD 20852-1790, http://www.usp.org.
3.2.2.1 Discussion—
The coefficient of variation (relative standard deviation) may be substituted for the standard deviation where applicable.
3.2.3 acceptance region, n—the set of values of parameter estimates (that is, sample mean and standard deviation) where
confidence limits attain a prescribed lower bound on the probability of passing a lot acceptance procedure.
3.2.4 confidence level, C, n—the prescribed overall level for calculating the uncertainty region of the parameters from the
sample estimates.
3.2.4.1 Discussion—
The preset confidence level is stated as a percentage, for example, 100 (1 – α) = 95 %, where α is a risk that is allocated to the two
parameters being estimated.
3.2.5 lower probability bound, LB, n—the nominal probability of passing the UDU test for a given set of parameter estimates.
3.2.6 multiple-stage acceptance procedure, n—a procedure that involves more than one stage of sampling and testing a given
quality characteristic with one or more acceptance criteria per stage.
3.2.7 representative sample, n—a sample that consists of a number of units that are drawn based on rational criteria such as
random sampling and intended to assure that the sample accurately portrays the material being sampled
3.2.8 sampling plan, n—scheme for selecting dosage units from locations within a batch for testing purposes.
3.2.8.1 Discussion—
In this standard, a single dosage unit is selected from each batch location.
3.2.9 uniformity of dosage units, UDU, n—the degree of uniformity in the amount of the drug substance among dosage units.
3.2.9.1 Discussion—
The requirements of the UDU test apply to each drug substance in dosage units containing one or more drug substances, unless
otherwise specified. The uniformity improves as the variability decreases.
4. Significance and Use
4.1 The methodology was originally developed (1-4) for use in drug content uniformity and dissolution but has general
application to any multistage test with multiple acceptance criteria. Practice E2709 summarizes the statistical aspects of this
methodology. This practice applies the general methodology of Practice E2709 specifically to the UDU test.
The boldface numbers in parentheses refer to a list of references at the end of this standard.
E2810 − 11 (2017)
4.1.1 While other methods can be used to estimate the probability of passing the UDU test, they are outside the scope of this
practice.
4.2 The UDU test procedure describes a two-stage sampling test, where at each stage one can pass or continue testing, and the
decision to fail is deferred until the second stage. At each stage there are acceptance criteria on the test results as outlined in Table
1.
4.3 The UDU test is a market standard. The USP General Notices include the following statement about compendial standards.
“The similarity to statistical procedures may seem to suggest an intent to make inference to some larger group of units, but in all
cases, statements about whether the compendial standard is met apply only to the units tested.” Therefore, the UDU procedure is
not intended for inspecting uniformity of finished product for lot/batch release or as a lot inspection procedure.
4.3.1 The UDU test defines a product requirement to be met at release and throughout the shelf-life of the product.
4.3.2 Passing the UDU test once does not provide statistical assurance that a batch of drug product meets specified statistical
quality control criteria.
4.4 This practice provides a practical specification that may be applied when uniformity of dosage units is required. An
acceptance region for the mean and standard deviation of a set of test results from the lot is defined such that, at a prescribed
confidence level, the probability that a future sample from the lot will pass the UDU test is greater than or equal to a prespecified
lower probability bound. Having test results fall in the acceptance region provides assurance that a sample would pass the UDU
test with at least the specified lower bound probability. This procedure does not account for any decrease in potency during the
shelf life, which could affect the ability to meet the UDU test requirements.
4.5 This practice can be used as an element for process demonstration or validation, continuous process verification, in-process
testing, or lot release (acceptance). As the circumstances and available information vary in these different application areas, this
practice does not prescribe a specific target, sample size, lower probability bound, or confidence level. These must be prospectively
selected by the user and may be different from those used in the acceptance limit tables provided in this practice.
5. Procedure
5.1 Generating The Acceptance Limit Table:Generating The Acceptance Limit Table:
5.1.1 The general procedure that generates the acceptance limit tables is described in Practice E2709 and the specific procedure
for application to the UDU test is described in the literature (4). A simplified description on the construction and use of these tables
TABLE 1 Uniformity of Dosage Units Test Procedure
NOTE 1—All measurements of dosage units and criteria values are in
percentage label claim (%LC). At each stage calculate the sample average,
X—, and the sample standard deviation, s.
Number
Stage Pass Stage If:
Tested
S 10 |M – X¯| + 2.4 s ≤ 15.0, where M is
defined below.
S 20 (1) |M – X¯| + 2.0 s ≤ 15.0, using
all 30 results (S + S ).
1 2
(2) No dosage unit is outside the
maximum allowed range of
0.75 * M to 1.25 * M.
M is defined as follows:
If T is less than or equal to 101.5 %LC, and
(1) If X¯ is less than 98.5 %LC, then
M = 98.5 %LC.
(2) If X¯ is between 98.5 and
101.5 %LC, then M = X¯.
(3) If X¯ is greater than 101.5 %LC,
then M = 101.5 %LC.
If T is greater than 101.5 %LC, and
(1) If X¯ is less than 98.5 %LC, then
M = 98.5 %LC.
(2) If X¯ is between 98.5 and T, then
M = X¯.
(3) If X¯ is greater than T, then
M = T.
T is the target content per dosage unit at the time of manufacture, ex-
pressed as %LC. Unless otherwise specified in the individual monograph, T
is 100.0 %LC.
E2810 − 11 (2017)
is given in this section. A computer program is required to generate the tables given a target T as a percentage of label claim (LC),
a lower probability bound LB, a confidence level C, and a sample size n.
5.1.2 The first step is to determine the acceptable parameter region. On a two-dimensional content space consisting of the true
mean (μ) on the horizontal axis and standard deviation (σ) on the vertical axis the upper boundary of this region is defined by a
contour, a curve that is concave downward and depicted by the solid curve in Fig. 1. The contour is determined by the LB
probability and the Target under the assumption that the dosage unit content is normally distributed. The acceptable parameter
region is the set of points on or below the contour. Any (μ, σ) pair in the acceptable region would pass the UDU test with a
probability of at least the LB.
5.1.3 The second step is to generate the acceptance limit curve. The sample mean (X¯) and sample standard deviation (s)
estimate the population parameters μ and σ within C % confidence limits as chosen by the user. The joint confidence region for
μ and σ (5) has the shape of an inverted triangle around a (X¯,s) pair as depicted in Fig. 1 with the lowest vertex at (X¯, 0). A value
of X¯ is selected starting with s = 0, then the confidence region is expanded by increasing s until one of the upper vertices just
touches the acceptable parameter region. The size of the confidence region is determined by C and n. This value of s defines a
point on the acceptance limit curve at (X¯,s). Additional selections of X¯ then generate the acceptance limit curve, as depicted as
dotted lines in Fig. 1. Acceptance limit curves are shown for n = 10 and n = 100, illustrating that the acceptance limits approach
the acceptable parameter region with increasing sample size.
5.1.4 Computer programs have been developed for generating acceptance limit tables, but these may not be available to all
practitioners. This practice contains four acceptance limit tables for many practical use situations.
5.2 Using the Acceptance Limit Tables in This Practice:
5.2.1 In each table acceptance limits on the standard deviation are given for means ranging 90–110 % of LC in increments of
0.2 %LC for sample sizes ranging from n = 10 to n = 500. In all tables the target is set at T = 100 %LC, so the acceptance limits
for standard deviations are symmetrical around 100 %LC. This target is also required for interchangeability across the ICH regions
(6).
NOTE 1—All points below the lower bound contour have higher than a 95 % chance of passing UDU test if mean and standard deviation are known.
All points below the acceptance region contours pass the associated acceptance limit table for n = 100 and n = 10.
ULS is the upper confidence limit for σ.
Z is a standard normal critical value.
FIG. 1 Example of Simultaneous Confidence Interval with 95 % Lower Bound and Acceptance Regions
E2810 − 11 (2017)
5.2.1.1 At the confidence level of C = 95 % used often in the regulatory arena, three levels of the probability lower bound are
provided: LB = 90 % (Table 2), LB = 95 % (Table 3) and LB = 99 % (Table 4). These provide 90 %, 95 %, and 99 % coverage,
respectively, of the population of dosage units under consideration. The usual coverage is 95 %.
5.2.1.2 Table 5 is provided at C = 90 % and LB = 95 % for comparison with Table 3 to demonstrate the effect of a lower
confidence level.
NOTE 1—Tables can also be generated for other choices for ranges of means, such as 85.1 to 114.9 %LC, or for other sample sizes.
TABLE 2 Acceptance Limits on Sample Standard Deviation (%LC) for
T = 100 %LC, C = 95 %, LB = 90 %LC
Sample Average Sample Size (n)
(%LC) 10 30 40 50 60 80 100 120 150 200 500
100.0 2.91 4.36 4.65 4.84 4.99 5.19 5.33 5.43 5.54 5.66 5.93
99.8 or 100.2 2.88 4.31 4.59 4.79 4.94 5.14 5.28 5.38 5.50 5.62 5.91
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.