ASTM D2460-97
(Test Method)Standard Test Method for Alpha-Particle-Emitting Isotopes of Radium in Water
Standard Test Method for Alpha-Particle-Emitting Isotopes of Radium in Water
SCOPE
1.1 This test method covers the separation of dissolved radium from water for the purpose of measuring its radioactivity. While all radium isotopes are included, the test method is limited to alpha-emitting radioisotopes by choice of radiation detector. The most important of these isotopes are radium-223, radium-224, and radium-226. The lower limit of concentration to which this test method is applicable is 1 pCi/L; it may be applied to higher concentration by reduction of sample size.
1.2 This test method may be used for absolute measurements by calibrating with a suitable alpha emitting radioisotope such as radium-226, or for relative methods by comparing measurements with each other. Mixtures of radium isotopes may be reported as equivalent radium-226. Information is also provided from which the relative contributions of radium isotopes may be calculated.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.> For a specific precautionary statement, see Section 9.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation:D2460–97
Standard Test Method for
Alpha-Particle-Emitting Isotopes of Radium in Water
This standard is issued under the fixed designation D 2460; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 3648 Practices for the Measurement of Radioactivity
1.1 This test method covers the separation of dissolved
3. Terminology
radium from water for the purpose of measuring its radioac-
3.1 Definition:
tivity. Although all radium isotopes are separated, the test
3.1.1 For definitions of terms used in this standard, see
method is limited to alpha-particle-emitting isotopes by choice
Terminology C 859 and D 1129. For terms not included in
ofradiationdetector.Themostimportantoftheseradioisotopes
these, reference may be made to other published glossaries (1,
are radium-223, radium-224, and radium-226. The lower limit
2).
of concentration to which this test method is applicable is 3.7
–2
3 10 Bq/L (1 pCi/L).
4. Summary of Test Method
1.2 This test method may be used for absolute measure-
4.1 Radium is collected from the water by coprecipitation
ments by calibrating with a suitable alpha-emitting radioiso-
with mixed barium and lead sulfates. The barium and lead
tope such as radium-226, or for relative methods by comparing
carriers are added to a solution containing alkaline citrate ion
measurements with each other. Mixtures of radium isotopes
which prevents precipitation until interchange has taken place.
may be reported as equivalent radium-226. Information is also
Sulfuric acid is then used to precipitate the sulfates, which are
provided from which the relative contributions of radium
purified by nitric acid washes. The precipitate is dissolved in
isotopes may be calculated.
ammoniacal EDTA. The barium and radium sulfates are
1.3 This standard does not purport to address all of the
reprecipitated by the addition of acetic acid, thereby separating
safety concerns, if any, associated with its use. It is the
them from lead and other radionuclides. The precipitate is
responsibility of the user of this standard to establish appro-
dried on a planchet weighed to determine the chemical yield,
priate safety and health practices and determine the applica-
and alpha-counted to determine the total disintegration rate of
bility of regulatory limitations prior to use. For a specific
alpha-particle-emitting radium isotopes. This procedure is
precautionary statement, see Section 9.
based upon published ones (3, 4).
2. Referenced Documents
5. Significance and Use
2.1 ASTM Standards:
5.1 Radium is one of the most radiotoxic elements. Its
C 859 Terminology Relating to Nuclear Materials
3 isotope of mass 226 is the most hazardous because of its long
D 1129 Terminology Relating to Water
half-life.The isotopes 223 and 224, although not as hazardous,
D 1193 Specification for Reagent Water
are of some concern in appraising the quality of water.
D 1943 Test Method for Alpha Particle Radioactivity of
5.2 The alpha-particle-emitting isotopes of radium other
Water
than that of mass 226 may be determined by difference if
D 2777 Practice for Determination of Precision and Bias of
radium-226 is measured separately, such as by Test Method
Applicable Methods of Committee D-19 on Water
3 D 3454. Note that one finds radium-226 and -223 together in
D 3370 Practices for Sampling Water
4 variable proportions (5, 6), but radium-224 does not normally
D 3454 Test Method for Radium-226 in Water
occur with them. Thus, radium-223 often may be determined
by simply subtracting the radium-226 content from the total:
This test method is under the jurisdiction ofASTM Committee D-19 on Water and if radium-226 and -223 are low, radium-224 may be
andisthedirectresponsibilityofSubcommitteeD19.04onMethodsofRadiochemi-
determined directly. The determination of a single isotope in a
cal Analysis.
mixture is less precise than if it occurred alone.
Current edition approved Aug 10, 1997. Published October 1997. Originally
issued 1966. Replaces D 2460–66 T. Last previous edition D 2460–90.
Annual Book of ASTM Standards, Vol 12.01.
3 5
Annual Book of ASTM Standards, Vol 11.01. The boldface numbers in parentheses refer to a list of references at the end of
Annual Book of ASTM Standards, Vol 11.02. this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2460–97
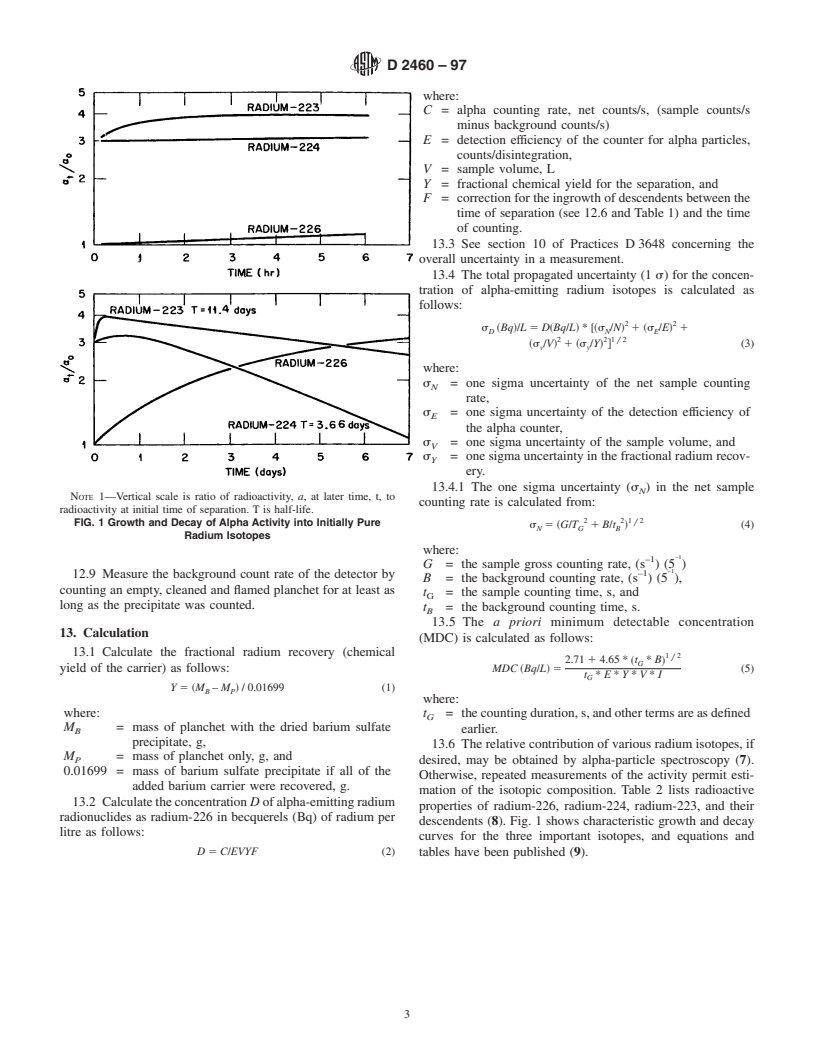
TABLE 1 Growth of Alpha Activity into Initially Pure Radium-226
6. Interferences
Time, h Correction, F
6.1 A barium content in the sample exceeding 0.2 mg will
0 1.0000
cause a falsely high chemical yield.
1 1.0160
2 1.0363
7. Apparatus
3 1.0580
7.1 For suitable gas-flow proportional or alpha-scintillation 4 1.0798
5 1.1021
counting equipment, refer to Test Method D 1943.
6 1.1238
24 1.4892
8. Reagents
48 1.9054
72 2.2525
8.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
all reagents shall conform to the specifications of the Commit-
10. Sampling
tee onAnalytical Reagents of theAmerican Chemical Society,
where such specifications are available. Other grades may be 10.1 Collect the sample in accordance with Practices
used, provided it is first ascertained that the reagent is of
D 3370 as applicable.
sufficiently high purity to permit its use without lessening the
10.2 Sample 1 L, or a smaller volume, provided that it is
precision, or increasing the bias, of the determination.
estimated to contain from 3.7 to 370 Bq (100 to 10 00 pCi) of
8.2 Purity of Water—Unless otherwise indicated, references
radium. Add 10 mL of HNO /L of sample.
towatershallbeunderstoodtomeanreagentwaterconforming
11. Calibration and Standardization
to Specification D 1193, Type III.
8.3 Radioactivity Purity Of Reagents—shallbesuchthatthe 11.1 For absolute counting, the alpha-particle detector must
measured results of blank samples do not exceed the calculated be calibrated to obtain the ratio of count rate to disintegration
probable error of the measurement or are within the desired rate.UseNISTtraceableradium-226standards.Analyzetwoor
precision. more portions of such solution, containing known disintegra-
8.4 Acetic Acid, Glacial (sp gr 1.05). tion rates, in accordance with Section 12. After counting,
8.5 Ammonium Hydroxide (sp gr 0.90)—Concentrated am- correct the measured activity for chemical yield, and calculate
monium hydroxide (NH OH). the efficiency, E (see Section 13), as the ratio of the observed
8.6 Ammonium Hydroxide (1+1) Mix 1 volume of concen- counting rate to the known disintegration rate.
trated ammonium hydroxide (NH OH, sp gr 0.90) with 1
12. Procedure
volume of water.
12.1 Add to a measured volume of sample 5 mL of citric
8.7 Barium Nitrate Carrier Solution (10 mg Ba/mL)—
acid and make alkaline (pH > 7.0) with NH OH. Confirm the
Dissolve 1.90 g of barium nitrate (Ba(NO ) ) in water and
3 2 4
alkalinity with pH-indicating paper or strip. Add 2 mL of lead
dilute to 100 mL.
carrier and 1.00 mL of barium carrier, and mix.
8.8 Citric Acid Solution (350 g/L)—Dissolve 350 g of citric
acid (anhydrous) in water and dilute to 1 L. 12.2 Heat to boiling and add 10 drops of methyl orange
pH-indicator solution. With stirring, add H SO (1 + 1) until
8.9 Disodium Ethylendiamine Tetraacetate Solution (93
2 4
g/L)—Dissolve 93 g of disodium ethylenediamine tetraacetate the solution becomes pink, then add 5 drops more.
12.3 Digest the precipitate with continued heating for 10
dihydrate in water and dilute to 1 L.
8.10 Lead Nitrate Carrier Solution (104 mg Pb/mL)— min. Let cool and collect the precipitate in a centrifuge tube.
When large volumes are handled, collection will be facilitated
Dissolve 33.2 g of lead nitrate (Pb(NO ) ) in water and dilute
3 2
to 200 mL. by first letting the precipitate settle, and then decanting most of
the clear liquid. Centrifuge then discard the supernatant liquid.
8.11 Methyl Orange Indicator Solution—Dissolve 1.0 g of
methyl orange in water and dilute to 1 L. 12.4 Wash the precipitate with 10 mL of HNO , centrifuge,
and discard the washings. Repeat this wash the precipitate.
8.12 Nitric Acid (sp gr 1.42)—Concentrated nitric acid
(HNO ). 12.5 Dissolve the precipitate in 10 mL of water, 10 mL of
EDTA solution, and 4 mL of NH OH (1 + 1). Warm if
8.13 Sulfuric Acid (1 + 1)—Cautiously add with stirring 1
necessary to effect dissolution.
volume of concentrated sulfuric acid (H SO , sp gr 1.84) to 1
2 4
12.6 Reprecipitate barium sulfate (BaSO ) by the dropwise
volume of water.
additionofaceticacid,thenadd3dropsmore.Recordthetime.
9. Safety Precautions
Centrifuge, then discard the supernatant liquid. Add 10 mL of
9.1 When diluting concentrated acids, always use safety
water, mix well, centrifuge, and discard the supernatant liquid.
glasses and protective clothing, and add the acid to the water.
12.7 Clean, flame, cool, and weigh a stainless steel planchet
that fits the alpha-particle counter being used. Transfer the
6 precipitate to the planchet with a m
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.