ASTM F564-02(2006)e1
(Specification)Standard Specification and Test Methods for Metallic Bone Staples
Standard Specification and Test Methods for Metallic Bone Staples
ABSTRACT
This specification covers characterization of the design and mechanical function of metallic staples used in the internal fixation of the muscular skeletal system. It is not the intention of this specification to describe or specify specific designs for metallic bone staples. Different test methods shall be performed in order to determine the following mechanical properties of metallic bone staples: bending fatigue, pull-out fixation strength, soft tissue fixation strength, and elastic static bending.
SIGNIFICANCE AND USE
A1.3.1 This test method is used to determine the fatigue resistance of metallic bone staples when subjected to repetitive loading for large numbers of cycles. This information may also be useful for comparing the effect of variations in staple material, geometry, surface condition, or placement under certain circumstances.
A1.3.2 It is essential that uniform fatigue practices be established in order that such basic fatigue data be comparable and reproducible and can be correlated among laboratories.
A1.3.3 The results of fatigue tests are suitable for direct application to design only when the service conditions parallel the test conditions exactly. This test method may not be appropriate for all types of bone staple applications. The user is cautioned to consider the appropriateness of the test method in view of the materials being tested and their potential application.
SCOPE
1.1 This specification covers characterization of the design and mechanical function of metallic staples used in the internal fixation of the muscular skeletal system. It is not the intention of this specification to describe or specify specific designs for metallic bone staples.
1.2 This specification includes the following four test methods for measuring mechanical properties of metallic bone staples:
1.2.1 Test Method for Constant Amplitude Bending Fatigue Tests of Metallic Bone Staples—Annex A1.
1.2.2 Test Method for Pull-Out Fixation Strength of Metallic Bone Staples—Annex A2.
1.2.3 Test Method for Soft Tissue Fixation Strength of Metallic Bone Staples—Annex A3.
1.2.4 Test Method for Elastic Static Bending of Metallic Bone Staples—Annex A4.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
A1.1.1 This test method covers procedures for the performance of constant amplitude fatigue testing of metallic staples used in internal fixation of the musculoskeletal system. This test method may be used when testing in air at ambient temperature or in an aqueous or physiological solution.
A1.1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
A1.1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
A2.1.1 This test method covers testing of the hard tissue pull-out fixation strength of metallic staples used in the internal fixation of the musculoskeletal system. This test method may be used with physiologic bone or a synthetic substitute. It may also be used when testing in an aqueous or physiological solution.
A2.1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
A2.1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate saf...
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: F564 – 02 (Reapproved 2006)
Standard Specification and Test Methods for
Metallic Bone Staples
ThisstandardisissuedunderthefixeddesignationF564;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Units information was editorially corrected in September 2009.
1. Scope E122 Practice for Calculating Sample Size to Estimate,
With Specified Precision, the Average for a Characteristic
1.1 This specification covers characterization of the design
of a Lot or Process
and mechanical function of metallic staples used in the internal
E467 Practice for Verification of Constant Amplitude Dy-
fixation of the muscular skeletal system. It is not the intention
namic Forces in an Axial Fatigue Testing System
of this specification to describe or specify specific designs for
F75 Specification for Cobalt-28 Chromium-6 Molybdenum
metallic bone staples.
Alloy Castings and Casting Alloy for Surgical Implants
1.2 This specification includes the following four test meth-
(UNS R30075)
ods for measuring mechanical properties of metallic bone
F86 Practice for Surface Preparation and Marking of Me-
staples:
tallic Surgical Implants
1.2.1 Test Method for ConstantAmplitude Bending Fatigue
F382 Specification and Test Method for Metallic Bone
Tests of Metallic Bone Staples—Annex A1.
Plates
1.2.2 Test Method for Pull-Out Fixation Strength of Metal-
F565 Practice for Care and Handling of Orthopedic Im-
lic Bone Staples—Annex A2.
plants and Instruments
1.2.3 Test Method for Soft Tissue Fixation Strength of
F601 Practice for Fluorescent Penetrant Inspection of Me-
Metallic Bone Staples—Annex A3.
tallic Surgical Implants
1.2.4 Test Method for Elastic Static Bending of Metallic
F629 Practice for Radiography of Cast Metallic Surgical
Bone Staples—Annex A4.
Implants
1.3 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this
3. Finish
standard.
3.1 Staples conforming to this specification shall be finished
1.4 This standard does not purport to address all of the
and identified in accordance with Practice F86, as appropriate.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4. Inspection Practices
priate safety and health practices and determine the applica-
4.1 Staples made in accordance with Specification F75
bility of regulatory limitations prior to use.
should be inspected in accordance with Practice F601 or
X-rayed in accordance with Practice F629.
2. Referenced Documents
2.1 ASTM Standards:
5. Care and Handling
E4 Practices for Force Verification of Testing Machines
5.1 Staples should be cared for and handled in accordance
with Practice F565, as appropriate.
This specification is under the jurisdiction of ASTM Committee F04 on
Medical and Surgical Materials and Devices and is the direct responsibility of 6. Keywords
Subcommittee F04.21 on Osteosynthesis.
6.1 bendingtest;bonefixation;fatiguetest;fixationdevices;
Current edition approved March 1, 2006. Published April 2006. Originally
metallic bone staples; orthopaedic medical devices; pullout
approved in 1985. Last previous edition approved in 2002 as F564 – 02. DOI:
10.1520/F0564-02R06E01.
test; soft tissue fixation; surgical implants
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
F564 – 02 (2006)
ANNEXES
(Mandatory Information)
A1. TEST METHOD FOR CONSTANT AMPLITUDE BENDING FATIGUE TESTS OF METALLIC BONE STAPLES
A1.1 Scope are fitted into fixation holes in each block with minimal
clearance to restrict bending of the staple within the hole. The
A1.1.1 This test method covers procedures for the perfor-
staple is fixed securely in the block using a moldable filling or
mance of constant amplitude fatigue testing of metallic staples
grouting agent. The extension design should minimize the
used in internal fixation of the musculoskeletal system. This
weight to reduce the influence on the staple while maintaining
test method may be used when testing in air at ambient
sufficient stiffness to transfer the load to the staple without
temperature or in an aqueous or physiological solution.
undesirable deflection. Holes for pin and clevis fixation are
A1.1.2 The values stated in SI units are to be regarded as
optional (see Figs. A1.1-A1.3).
standard. No other units of measurement are included in this
standard.
NOTE A1.1—Variations in fixation hole configuration may be required
A1.1.3 This standard does not purport to address all of the
for staple legs with noncircular cross sections. Also, it is necessary to
provide a gap between the underside of the staple bridge and edge of the
safety concerns, if any, associated with its use. It is the
staple extender in most cases. This is necessary to eliminate contact
responsibility of the user of this standard to establish appro-
between the staple bridge (or other bridge features such as tissue spikes)
priate safety and health practices and determine the applica-
and the staple extender. However, this gap should be standardized within
bility of regulatory limitations prior to use.
any test group as required.
A1.4.2.2 4-Point Bend Fixture—A standard or modified
A1.2 Summary of Test Method
bending fixture that produces pure bending in the staple
A1.2.1 Metallic bone staples are tested under bending loads
withoutappreciableshearortorsionwhenusedtoapplyloadto
until the specimen fails or a predetermined number of cycles
the staple through the staple extensions.
has been applied to it. Bending tests may be performed in one
A1.4.2.3 Pin and Clevice Fixture—A standard or modified
of two modes: either pure, in-plane bending; or tension (or
fixture used to apply a distractive or compressive load to the
compression) combined with in-plane bending. Tests using
staple through the staple extensions to produce bending in the
either of these methods may be conducted at ambient condi-
staple similar to that seen in vivo.
tions or in aqueous or physiological solutions (at either room
A1.4.3 Filling or Grouting Agent—A stiff, moldable filler,
temperature or 37°C).
suchasepoxy,acryliccement,oralow-meltingpointalloy(for
example,Wood’smetal)usedtosecurethestaplelegwithinthe
A1.3 Significance and Use
staple extension.
A1.3.1 This test method is used to determine the fatigue
A1.4.4 Aqueous Solution—Tapwater,distilledwater,physi-
resistance of metallic bone staples when subjected to repetitive
ological saline, or similar aqueous solutions, used to immerse
loading for large numbers of cycles.This information may also
the test specimens fully during the test.
be useful for comparing the effect of variations in staple
material, geometry, surface condition, or placement under
certain circumstances.
A1.3.2 It is essential that uniform fatigue practices be
established in order that such basic fatigue data be comparable
and reproducible and can be correlated among laboratories.
A1.3.3 The results of fatigue tests are suitable for direct
application to design only when the service conditions parallel
the test conditions exactly. This test method may not be
appropriate for all types of bone staple applications. The user
is cautioned to consider the appropriateness of the test method
in view of the materials being tested and their potential
application.
A1.4 Apparatus
A1.4.1 Testing Machines,conformingtotherequirementsof
Practices E4 and E467. The loads used for determining
strengths shall be within the loading range of the testing
machine as defined in Practices E4 and E467.
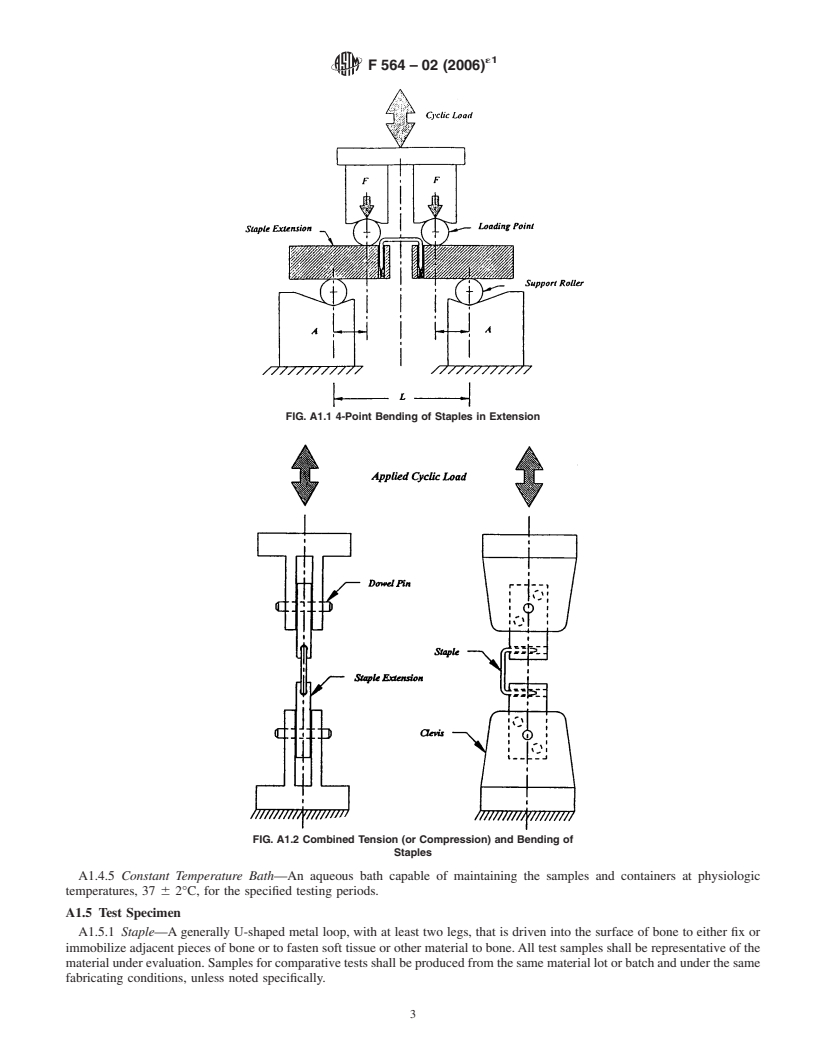
A1.4.2 Gripping Devices:
A1.4.2.1 Staple Extensions—Pairs of specially designed
metal blocks that permit the holding of individual staples for
theapplicationofbendingfatigueloads.Thelegsofeachstaple FIG. A1.1 4-Point Bending of Staples in Extension
´1
F564 – 02 (2006)
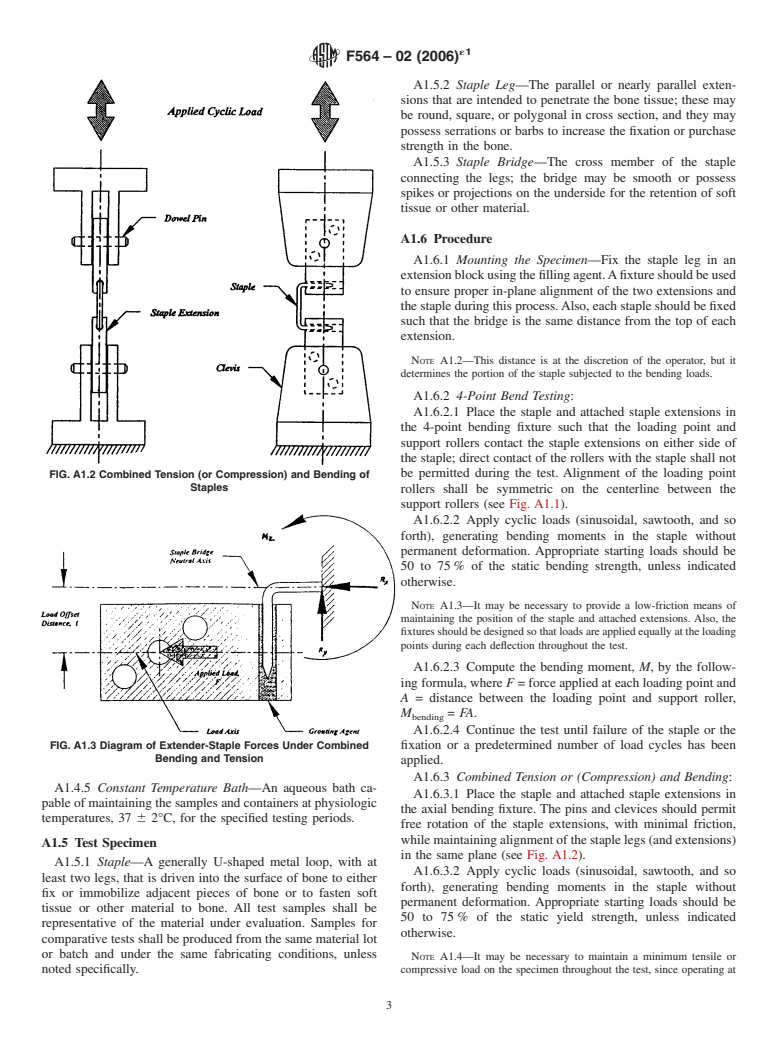
A1.5.2 Staple Leg—The parallel or nearly parallel exten-
sions that are intended to penetrate the bone tissue; these may
be round, square, or polygonal in cross section, and they may
possess serrations or barbs to increase the fixation or purchase
strength in the bone.
A1.5.3 Staple Bridge—The cross member of the staple
connecting the legs; the bridge may be smooth or possess
spikes or projections on the underside for the retention of soft
tissue or other material.
A1.6 Procedure
A1.6.1 Mounting the Specimen—Fix the staple leg in an
extensionblockusingthefillingagent.Afixtureshouldbeused
to ensure proper in-plane alignment of the two extensions and
the staple during this process.Also, each staple should be fixed
such that the bridge is the same distance from the top of each
extension.
NOTE A1.2—This distance is at the discretion of the operator, but it
determines the portion of the staple subjected to the bending loads.
A1.6.2 4-Point Bend Testing:
A1.6.2.1 Place the staple and attached staple extensions in
the 4-point bending fixture such that the loading point and
support rollers contact the staple extensions on either side of
the staple; direct contact of the rollers with the staple shall not
be permitted during the test. Alignment of the loading point
FIG. A1.2 Combined Tension (or Compression) and Bending of
Staples
rollers shall be symmetric on the centerline between the
support rollers (see Fig. A1.1).
A1.6.2.2 Apply cyclic loads (sinusoidal, sawtooth, and so
forth), generating bending moments in the staple without
permanent deformation. Appropriate starting loads should be
50 to 75 % of the static bending strength, unless indicated
otherwise.
NOTE A1.3—It may be necessary to provide a low-friction means of
maintaining the position of the staple and attached extensions. Also, the
fixtures should be designed so that loads are applied equally at the loading
points during each deflection throughout the test.
A1.6.2.3 Compute the bending moment, M, by the follow-
ing formula, where F = force applied at each loading point and
A = distance between the loading point and support roller,
M = FA.
bending
A1.6.2.4 Continue the test until failure of the staple or the
FIG. A1.3 Diagram of Extender-Staple Forces Under Combined fixation or a predetermined number of load cycles has been
Bending and Tension
applied.
A1.6.3 Combined Tension or (Compression) and Bending:
A1.4.5 Constant Temperature Bath—An aqueous bath ca-
A1.6.3.1 Place the staple and attached staple extensions in
pable of maintaining the samples and containers at physiologic
the axial bending fixture. The pins and clevices should permit
temperatures, 37 6 2°C, for the specified testing periods.
free rotation of the staple extensions, with minimal friction,
whilemaintainingalignmentofthestaplelegs(andextensions)
A1.5 Test Specimen
in the same plane (see Fig. A1.2).
A1.5.1 Staple—A generally U-shaped metal loop, with at
A1.6.3.2 Apply cyclic loads (sinusoidal, sawtooth, and so
least two legs, that is driven into the surface of bone to either
forth), generating bending moments in the staple without
fix or immobilize adjacent pieces of bone or to fasten soft
permanent deformation. Appropriate starting loads should be
tissue or other material to bone. All test samples shall be
50 to 75 % of the static yield strength, unless indicated
representative of the material under evaluation. Samples for
otherwise.
comparative tests shall be produced from the same material lot
or batch and under the same fabricating conditions, unless
NOTE A1.4—It may be necessary to maintain a minimum tensile or
noted specifically. compressive load on the specimen throughout the test, since operating at
´1
F564 – 02 (2006)
or near zero load may result in either loss of machine control due to
A1.8.1.1 Staple Description—Type, size, special features
discontinuity in the load feedback loop or undesirable transient loading of
(barbs, spikes, and so forth), manufacturer, material, batch or
the staple.
lot number, and dimensions (including leg length, bridge
A1.6.3.3 Compute the bending moment in the staple bridge,
width, and length), as appropriate.
M, by the following formula, where F = force applied at each A1.8.1.2 Test Type—4-point or combined tension (or com-
center of each pin and L = distance between the load applica-
pression) and bending.
tion axis, that is, the pin center, and the neutral axis of the A1.8.1.3 Fixation Geometry—Load point separation dis-
staple bridge, M = FL (see Fig. A1.3).
tances (4-point bending), load offset distance (combined ten-
bending
sion and bending), staple bridge-extension distance, and so
NOTE A1.5—The application of this test method produces bending,
forth.
tensile(orcompressive),andshearstressesinthestaple.Thedirectionand
A1.8.1.4 Minimum and maximum cycle loads, test fre-
magnitudes of these stresses should be analyzed using superposition
theory or other suitable methods. quency(forexample,cycles/s),andforcingfunctiontype(sine,
ramp, saw tooth, and so forth).
A1.6.3.4 Continue the test until failure of the staple or the
A1.8.1.5 Bending moment, M (N-m).
fixation or a predetermined number of load cycles has been
A1.8.1.6 Load ratio, R, where R = minimum load/maximum
applied.
load.
A1.6.4 Stress Verification—It is recommended that strain
A1.8.1.7 Test Environment—Ambient air or physiological
gages(orextensometry)beusedtomeasurethebendingstrains
solution.
induced in the specimen. This is accomplished most easily on
A1.8.1.8 Number of cycles at failure or test termination
thestaplebridge,butitmaybepossibletoperformonaportion
(runout).
of the staple leg or at the leg-bridge junction under certain
A1.8.1.9 Location of fatigue fracture (if applicable).
circumstances and with certain staple designs. The recom-
A1.8.1.10 Reason for test termination, that is, staple failure,
mended technique is to strain gage the actual fatigue test
fixation failure, runout to specified cycle limit, and so forth.
specimens, if possible, provided that the installation of strain
gage will not influence the test results.
A1.9 Precision
A1.7 Test Termination
A1.9.1 Intralaboratory and interlaboratory reproducibility
have not been determined systematically.
A1.7.1 Continue the tests until the specimen fails or a
predetermined number of cycles has been applied to the
A1.10 Rationale (Nonmandatory Information)
specimen. Failure should be defined as co
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´1
Designation:F564–00 Designation: F 564 – 02 (Reapproved 2006)
Standard Specification and Test Methods for
Metallic Bone Staples
This standard is issued under the fixed designation F 564; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Units information was editorially corrected in September 2009.
1. Scope
1.1 This specification covers characterization of the design and mechanical function of metallic staples used in the internal
fixation of the muscular skeletal system. It is not the intention of this specification to describe or specify specific designs for
metallic bone staples.
1.2 This specification includes the following four test methods for measuring mechanical properties of metallic bone staples:
1.2.1 Test Method for Constant Amplitude Bending Fatigue Tests of Metallic Bone Staples—Annex A1.
1.2.2 Test Method for Pull-Out Fixation Strength of Metallic Bone Staples—Annex A2.
1.2.3 Test Method for Soft Tissue Fixation Strength of Metallic Bone Staples—Annex A3.
1.2.4 Test Method for Elastic Static Bending of Metallic Bone Staples—Annex A4.
1.3Unless otherwise indicated, the values stated in SI units are to be regarded as standard. The values given in parentheses are
given for information only.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
E4 Practices for Force Verification of Testing Machines
E 122 PracticeforChoiceofSampleSizetoEstimateaMeasureofQualityforaLotorProcessPracticeforCalculatingSample
Size to Estimate, With Specified Precision, the Average for a Characteristic of a Lot or Process
E 467 PracticeforVerificationofConstantAmplitudeDynamicLoadsForcesinanAxialLoadFatigueTestingMachine System
F75 SpecificationforCastCobalt-Chromium-MolybdenumAlloyforSurgicalImplantApplicationsSpecificationforCobalt-28
Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS R30075)
F86 Practice for Surface Preparation and Marking of Metallic Surgical Implants
F 382 Test Method for Static Bending Properties of Metallic Bone Plates Specification and Test Method for Metallic Bone
Plates
F 565 Standard Practice for Care and Handling of Orthopedic Implants and Instruments
F 601 Practice for Fluorescent Penetrant Inspection of Metallic Surgical Implants
F 629 Practice for Radiography of Cast Metallic Surgical Implants
3. Finish
3.1 Staples conforming to this specification shall be finished and identified in accordance with Practice F 86, as appropriate.
4. Inspection Practices
4.1 Staples made in accordance with Specification F 75 should be inspected in accordance with Practice F 601 or X-rayed in
accordance with Practice F 629.
ThisspecificationisunderthejurisdictionofASTMCommitteeF-04F04onMedicalandSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee
F04.21 on Osteosynthesis.
´1
Current edition approved May 10, 2000. Published August 2000. Originally published as F564–85. Last previous edition F564–85 (1997) .
Current edition approved March 1, 2006. Published April 2006. Originally approved in 1985. Last previous edition approved in 2002 as F 564 – 02.
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
, Vol 03.01.volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
F 564 – 02 (2006)
5. Care and Handling
5.1 Staples should be cared for and handled in accordance with Practice F 565, as appropriate.
6. Keywords
6.1 bending test; bone fixation; fatigue test; fixation devices; metallic bone staples; orthopaedic medical devices; pullout test;
soft tissue fixation; surgical implants
ANNEXES
(Mandatory Information)
A1. TEST METHOD FOR CONSTANT AMPLITUDE BENDING FATIGUE TESTS OF METALLIC BONE STAPLES
A1.1 Scope
A1.1.1 This test method covers procedures for the performance of constant amplitude fatigue testing of metallic staples used
in internal fixation of the musculoskeletal system. This test method may be used when testing in air at ambient temperature or in
an aqueous or physiological solution.
A1.1.2The values stated in SI units are to be regarded as the standard.
A1.1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
A1.1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
A1.2 Summary of Test Method
A1.2.1 Metallic bone staples are tested under bending loads until the specimen fails or a predetermined number of cycles has
beenappliedtoit.Bending tests may be performed inoneoftwomodes:eitherpure,in-planebending;ortension(or compression)
combined with in-plane bending. Tests using either of these methods may be conducted at ambient conditions or in aqueous or
physiological solutions (at either room temperature or 37°C).
A1.3 Significance and Use
A1.3.1 This test method is used to determine the fatigue resistance of metallic bone staples when subjected to repetitive loading
forlargenumbersofcycles.Thisinformationmayalsobeusefulforcomparingtheeffectofvariationsinstaplematerial,geometry,
surface condition, or placement under certain circumstances.
A1.3.2 It is essential that uniform fatigue practices be established in order that such basic fatigue data be comparable and
reproducible and can be correlated among laboratories.
A1.3.3 The results of fatigue tests are suitable for direct application to design only when the service conditions parallel the test
conditions exactly. This test method may not be appropriate for all types of bone staple applications. The user is cautioned to
consider the appropriateness of the test method in view of the materials being tested and their potential application.
A1.4 Apparatus
A1.4.1 Testing Machines, conforming to the requirements of Practices E 4 and E 467. The loads used for determining strengths
shall be within the loading range of the testing machine as defined in Practices E 4 and E 467.
A1.4.2 Gripping Devices:
A1.4.2.1 Staple Extensions—Pairs of specially designed metal blocks that permit the holding of individual staples for the
application of bending fatigue loads. The legs of each staple are fitted into fixation holes in each block with minimal clearance to
restrict bending of the staple within the hole. The staple is fixed securely in the block using a moldable filling or grouting agent.
The extension design should minimize the weight to reduce the influence on the staple while maintaining sufficient stiffness to
transfer the load to the staple without undesirable deflection. Holes for pin and clevis fixation are optional (see Figs.A1.1-A1.3).
NOTE A1.1—Variations in fixation hole configuration may be required for staple legs with noncircular cross sections. Also, it is necessary to provide
a gap between the underside of the staple bridge and edge of the staple extender in most cases. This is necessary to eliminate contact between the staple
bridge (or other bridge features such as tissue spikes) and the staple extender. However, this gap should be standardized within any test group as required.
A1.4.2.2 4-Point Bend Fixture—A standard or modified bending fixture that produces pure bending in the staple without
appreciable shear or torsion when used to apply load to the staple through the staple extensions.
A1.4.2.3 Pin and Clevice Fixture—Astandard or modified fixture used to apply a distractive or compressive load to the staple
through the staple extensions to produce bending in the staple similar to that seen in vivo.
A1.4.3 Filling or Grouting Agent—A stiff, moldable filler, such as epoxy, acrylic cement, or a low-melting point alloy (for
example, Wood’s metal) used to secure the staple leg within the staple extension.
A1.4.4 Aqueous Solution—Tapwater,distilledwater,physiologicalsaline,orsimilaraqueoussolutions,usedtoimmersethetest
specimens fully during the test.
´1
F 564 – 02 (2006)
FIG. A1.1 4-Point Bending of Staples in Extension
FIG. A1.2 Combined Tension (or Compression) and Bending of
Staples
A1.4.5 Constant Temperature Bath—An aqueous bath capable of maintaining the samples and containers at physiologic
temperatures, 37 6 2°C, for the specified testing periods.
A1.5 Test Specimen
A1.5.1 Staple—A generally U-shaped metal loop, with at least two legs, that is driven into the surface of bone to either fix or
immobilize adjacent pieces of bone or to fasten soft tissue or other material to bone.All test samples shall be representative of the
material under evaluation. Samples for comparative tests shall be produced from the same material lot or batch and under the same
fabricating conditions, unless noted specifically.
´1
F 564 – 02 (2006)
FIG. A1.3 Diagram of Extender-Staple Forces Under Combined
Bending and Tension
A1.5.2 Staple Leg—The parallel or nearly parallel extensions that are intended to penetrate the bone tissue; these may be round,
square, or polygonal in cross section, and they may possess serrations or barbs to increase the fixation or purchase strength in the
bone.
A1.5.3 Staple Bridge—The cross member of the staple connecting the legs; the bridge may be smooth or possess spikes or
projections on the underside for the retention of soft tissue or other material.
A1.6 Procedure
A1.6.1 Mounting the Specimen—Fix the staple leg in an extension block using the filling agent. A fixture should be used to
ensure proper in-plane alignment of the two extensions and the staple during this process. Also, each staple should be fixed such
that the bridge is the same distance from the top of each extension.
NOTE A1.2—This distance is at the discretion of the operator, but it determines the portion of the staple subjected to the bending loads.
A1.6.2 4-Point Bend Testing:
A1.6.2.1 Place the staple and attached staple extensions in the 4-point bending fixture such that the loading point and support
rollers contact the staple extensions on either side of the staple; direct contact of the rollers with the staple shall not be permitted
during the test. Alignment of the loading point rollers shall be symmetric on the centerline between the support rollers (see Fig.
A1.1).
A1.6.2.2 Apply cyclic loads (sinusoidal, sawtooth, etc.),and so forth), generating bending moments in the staple without
permanent deformation.Appropriate starting loads should be 50 to 75 % of the static bending strength, unless indicated otherwise.
NOTE A1.3—It may be necessary to provide a low-friction means of maintaining the position of the staple and attached extensions. Also, the fixtures
should be designed so that loads are applied equally at the loading points during each deflection throughout the test.
A1.6.2.3 Compute the bending moment, M, by the following formula, where F = force applied at each loading point and A =
distance between the loading point and support roller, M = FA.
bending
A1.6.2.4 Continue the test until failure of the staple or the fixation or a predetermined number of load cycles has been applied.
A1.6.3 Combined Tension or (Compression) and Bending:
A1.6.3.1 Place the staple and attached staple extensions in the axial bending fixture. The pins and clevices should permit free
rotation of the staple extensions, with minimal friction, while maintaining alignment of the staple legs (and extensions) in the same
plane (see Fig. A1.2).
A1.6.3.2 Apply cyclic loads (sinusoidal, sawtooth, etc.),and so forth), generating bending moments in the staple without
permanent deformation. Appropriate starting loads should be 50 to 75 % of the static yield strength, unless indicated otherwise.
NOTE A1.4—It may be necessary to maintain a minimum tensile or compressive load on the specimen throughout the test, since operating at or near
zero load may result in either loss of machine control due to discontinuity in the load feedback loop or undesirable transient loading of the staple.
A1.6.3.3 Computethebendingmomentinthestaplebridge, M,bythefollowingformula,where F=forceappliedateachcenter
of each pin and L = distance between the load application axis, that is, the pin center, and the neutral axis of the staple bridge,
M = FL (see Fig. A1.3).
bending
NOTE A1.5—The application of this test method produces bending, tensile (or compressive), and shear stresses in the staple. The direction and
magnitudes of these stresses should be analyzed using superposition theory or other suitable methods.
A1.6.3.4 Continue the test until failure of the staple or the fixation or a predetermined number of load cycles has been applied.
A1.6.4 Stress Verification—It is recommended that strain gages (or extensometry) be used to measure the bending strains
induced in the specimen. This is accomplished most easily on the staple bridge, but it may be possible to perform on a portion of
´1
F 564 – 02 (2006)
thestaplelegorattheleg-bridgejunctionundercertaincircumstancesandwithcertainstapledesigns.Therecommendedtechnique
is to strain gage the actual fatigue test specimens, if possible, provided that the installation of strain gage will not influence the
test results.
A1.7 Test Termination
A1.7.1 Continuethetestsuntilthespecimenfailsorapredeterminednumberofcycleshasbeenappliedtothespecimen.Failure
should be defined as complete separation, a crack visible at a specified magnification, a crack of certain dimensions, or by some
other criterion. State the criterion selected for defining failure when reporting the results.
A1.7.2 Atest shall be conside
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.