ASTM D5904-02(2017)
(Test Method)Standard Test Method for Total Carbon, Inorganic Carbon, and Organic Carbon in Water by Ultraviolet, Persulfate Oxidation, and Membrane Conductivity Detection
Standard Test Method for Total Carbon, Inorganic Carbon, and Organic Carbon in Water by Ultraviolet, Persulfate Oxidation, and Membrane Conductivity Detection
SIGNIFICANCE AND USE
5.1 This test method is used for determination of the carbon content of water from a variety of natural, domestic, and industrial sources. In its most common form, this test method is used to measure organic carbon as a means of monitoring organic pollutants in high purity and drinking water. These measurements are also used in monitoring waste treatment processes.
5.2 The relationship of TOC to other water quality parameters such as chemical oxygen demand (COD) and total oxygen demand (TOD) is described in the literature (6).
SCOPE
1.1 This test method covers the determination of total carbon (TC), inorganic carbon (IC), and total organic carbon (TOC) in water in the range from 0.5 to 30 mg/L of carbon. Higher levels may be determined by sample dilution. The test method utilizes ultraviolet-persulfate oxidation of organic carbon, coupled with a CO2 selective membrane to recover the CO2 into deionized water. The change in conductivity of the deionized water is measured and related to carbon concentration in the oxidized sample. Inorganic carbon is determined in a similar manner without the requirement for oxidation. In both cases, the sample is acidified to facilitate CO2 recovery through the membrane. The relationship between the conductivity measurement and carbon concentration is described by a set of chemometric equations for the chemical equilibrium of CO2, HCO3−, H+, and the relationship between the ionic concentrations and the conductivity. The chemometric model includes the temperature dependence of the equilibrium constants and the specific conductances.
1.2 This test method has the advantage of a very high sensitivity detector that allows very low detection levels on relatively small volumes of sample. Also, use of two measurement channels allows determination of CO2 in the sample independently of organic carbon. Isolation of the conductivity detector from the sample by the CO2 selective membrane results in a very stable calibration, with minimal interferences.
1.3 This test method was used successfully with reagent water spiked with sodium bicarbonate and various organic materials. It is the user's responsibility to ensure the validity of this test method for waters of untested matrices.
1.4 This test method is applicable only to carbonaceous matter in the sample that can be introduced into the reaction zone. The injector opening size generally limits the maximum size of particles that can be introduced.
1.5 In addition to laboratory analyses, this test method may be applied to on line monitoring.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D5904 − 02 (Reapproved 2017)
Standard Test Method for
Total Carbon, Inorganic Carbon, and Organic Carbon in
Water by Ultraviolet, Persulfate Oxidation, and Membrane
Conductivity Detection
This standard is issued under the fixed designation D5904; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.5 In addition to laboratory analyses, this test method may
be applied to on line monitoring.
1.1 This test method covers the determination of total
carbon (TC), inorganic carbon (IC), and total organic carbon 1.6 The values stated in SI units are to be regarded as
(TOC) in water in the range from 0.5 to 30 mg/L of carbon. standard. No other units of measurement are included in this
Higher levels may be determined by sample dilution. The test standard.
method utilizes ultraviolet-persulfate oxidation of organic
1.7 This standard does not purport to address all of the
carbon, coupled with a CO selective membrane to recover the
safety concerns, if any, associated with its use. It is the
CO into deionized water. The change in conductivity of the
2 responsibility of the user of this standard to establish appro-
deionized water is measured and related to carbon concentra-
priate safety, health, and environmental practices and deter-
tion in the oxidized sample. Inorganic carbon is determined in
mine the applicability of regulatory limitations prior to use.
asimilarmannerwithouttherequirementforoxidation.Inboth
1.8 This international standard was developed in accor-
cases,thesampleisacidifiedtofacilitateCO recoverythrough
dance with internationally recognized principles on standard-
the membrane. The relationship between the conductivity
ization established in the Decision on Principles for the
measurement and carbon concentration is described by a set of
Development of International Standards, Guides and Recom-
chemometric equations for the chemical equilibrium of CO ,
mendations issued by the World Trade Organization Technical
− +
HCO ,H , and the relationship between the ionic concentra-
Barriers to Trade (TBT) Committee.
tions and the conductivity. The chemometric model includes
the temperature dependence of the equilibrium constants and
2. Referenced Documents
the specific conductances. 2
2.1 ASTM Standards:
1.2 This test method has the advantage of a very high
D1129Terminology Relating to Water
sensitivity detector that allows very low detection levels on
D1192Guide for Equipment for Sampling Water and Steam
relatively small volumes of sample.Also, use of two measure-
in Closed Conduits (Withdrawn 2003)
ment channels allows determination of CO in the sample
D1193Specification for Reagent Water
independently of organic carbon. Isolation of the conductivity
D2777Practice for Determination of Precision and Bias of
detector from the sample by the CO selective membrane
Applicable Test Methods of Committee D19 on Water
results in a very stable calibration, with minimal interferences.
D3370Practices for Sampling Water from Closed Conduits
D5810Guide for Spiking into Aqueous Samples
1.3 This test method was used successfully with reagent
D5847Practice for Writing Quality Control Specifications
water spiked with sodium bicarbonate and various organic
for Standard Test Methods for Water Analysis
materials.Itistheuser’sresponsibilitytoensurethevalidityof
this test method for waters of untested matrices.
3. Terminology
1.4 This test method is applicable only to carbonaceous
3.1 Definitions:
matter in the sample that can be introduced into the reaction
3.1.1 For definitions of terms used in this standard, refer to
zone. The injector opening size generally limits the maximum
Terminology D1129.
size of particles that can be introduced.
1 2
This test method is under the jurisdiction ofASTM Committee D19 on Water For referenced ASTM standards, visit the ASTM website, www.astm.org, or
andisthedirectresponsibilityofSubcommitteeD19.06onMethodsforAnalysisfor contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Organic Substances in Water. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Feb. 1, 2017. Published February 2017. Originally the ASTM website.
approved in 1996. Last previous edition approved in 2009 as D5904 – 02 (2009). The last approved version of this historical standard is referenced on
DOI: 10.1520/D5904-02R17. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5904 − 02 (2017)
3.2 Definitions of Terms Specific to This Standard: 4. Summary of Test Method
3.2.1 inorganic carbon (IC), n—carbon in the form of
4.1 Fundamentals—Carbon can occur in water as inorganic
carbon dioxide, carbonate ion, or bicarbonate ion.
and organic compounds.This test method can be used to make
independent measurements of IC and TC and can also deter-
3.2.2 potassium hydrogen phthalate (KHP), n—KHC H O .
8 4 4
mineTOCasthedifferenceofTCandIC.IfICishighrelative
3.2.3 refractory material, n—that which cannot be oxidized
toTOCitisdesirabletouseavacuumdegassingunittoreduce
completely under the test method conditions.
the IC concentration as part of the measurement.Alternatively,
3.2.4 total carbon (TC), n—the sum of IC and TOC.
the IC can be removed by acidifying and sparging the sample
prior to injection into the instrument.
3.2.5 total organic carbon (TOC), n—carbon in the form of
organic compounds. 4.2 The basic steps of this test method are:
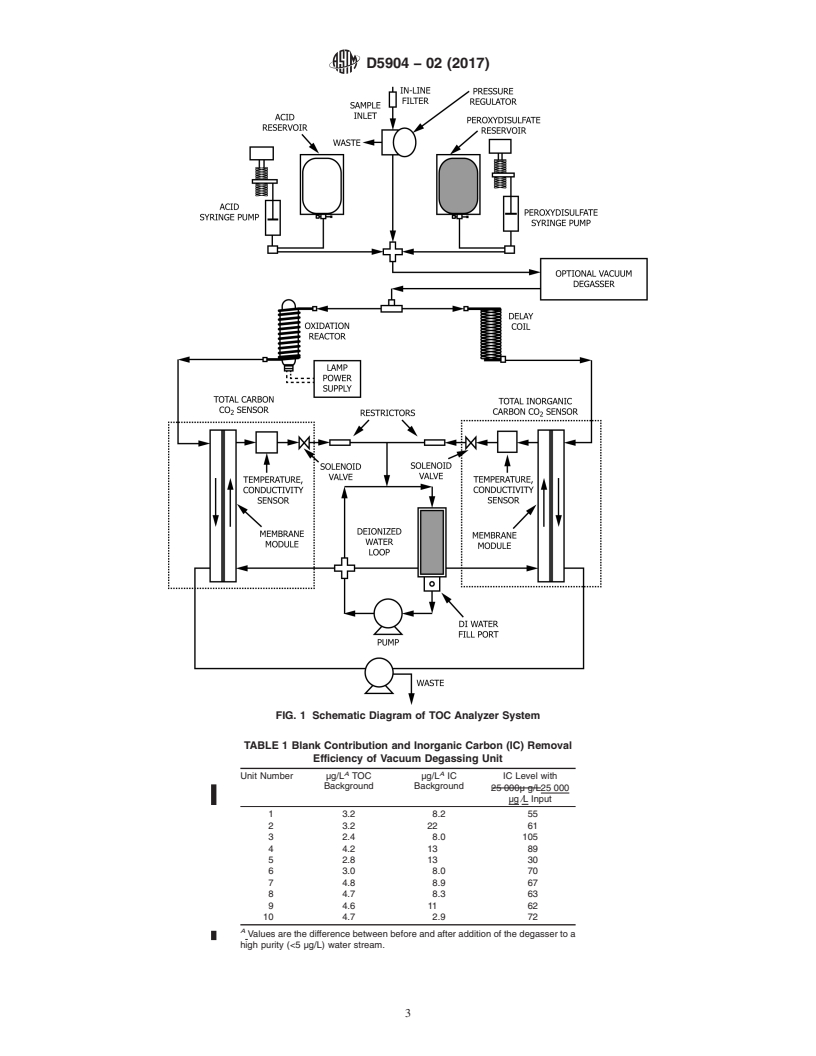
FIG. 1 Schematic Diagram of TOC Analyzer System
D5904 − 02 (2017)
4.2.1 Removal of IC, if desired, by vacuum degassing; 6.2 Chloride ion above 250 mg/L tends to interfere with
4.2.2 Conversion of remaining inorganic carbon to CO by oxidative reaction mechanisms in this test method. Follow
action of acid in both channels and oxidation of total carbon to manufacturer’s instructions for dealing with this problem.
CO by action of acid-persulfate, aided by ultraviolet (UV) Other interferences have been investigated and found to be
radiation in the TC channel; minimal under most conditions. Refer to the references for
4.2.3 Detection of CO that is swept out of the reactors by more information.
the liquid stream over membranes that allow the specific
6.3 Note that error will be introduced when the method of
passage of CO to high purity water where change in conduc-
difference is used to derive a relatively small level from two
tivity is measured; and
large levels. For example, a ground water high in IC and low
4.2.4 Conversion of the conductivity detector signal to a
inTOCwillgiveapoorerTOCvalueas(TC-IC)thanbydirect
display of carbon concentration in parts per million
measurement. In this case the vacuum degassing unit on the
(ppm=mg⁄L) or parts per billion (ppb=µg⁄L). The IC chan-
instrument should be used to reduce the concentration of IC
nel reading is subtracted from the TC channel to give a TOC
prior to measurement. Alternatively, the sample can be acidi-
reading. A diagram of suitable apparatus is given in Fig. 1.
fied and sparged prior to introduction into the instrument. Use
References (1-5) provide additional information on this test
ofthevacuumdegassingunitorspargingthesamplemaycause
method.
lossofvolatileorganiccompounds,thusyieldingavaluelower
than the true TOC level.
5. Significance and Use
6.4 Use of the vacuum degassing unit or sparging the
5.1 Thistestmethodisusedfordeterminationofthecarbon
sample may cause loss of volatile organic compounds, thus
content of water from a variety of natural, domestic, and
yielding a value lower than the true TOC level. At low TOC
industrial sources. In its most common form, this test method
levels,thedegassingunitmayintroduceameasurableTOCand
is used to measure organic carbon as a means of monitoring
IC background. The user should characterize the background
organic pollutants in high purity and drinking water. These
andperformanceofthedegassingmodulefortheirapplication.
measurements are also used in monitoring waste treatment
A removal efficiency of 97% of the inlet IC is considered
processes.
satisfactory. Table 1 provides typical IC removal performance
5.2 The relationship of TOC to other water quality param-
and background levels of the vacuum degassing unit.
eterssuchaschemicaloxygendemand(COD)andtotaloxygen
demand (TOD) is described in the literature (6).
7. Apparatus
6. Interferences and Limitations
7.1 Homogenizing Apparatus—Ahousehold blender is gen-
erally satisfactory for homogenizing immiscible phases in
6.1 The oxidation of dissolved carbon to CO is brought
water.
about at relatively low temperatures by the chemical action of
reactivespeciesproducedbyUV-irradiatedpersulfateions.Not
7.2 Apparatus for Carbon Determination—Atypical instru-
all suspended or refractory material may be oxidized under
ment consists of reagent and sample introduction mechanism,
these conditions; analysts should take steps to determine what
reaction vessel, detector, control system, and a display. Fig. 1
recovery is being obtained. This may be done by several
shows a diagram of such an arrangement.
methods: by rerunning the sample under more vigorous reac-
7.2.1 Vacuum degassing requires the manufacturer’s mod-
tion conditions; by analyzing the sample by an alternative
ule that includes a vacuum pump and a hollow fiber mem-
methodknowntoresultinfullrecovery;orbyspikingsamples
brane assembly. Use of this vacuum degasser will remove
with known refractories and determining recovery.
essentiallyallICaspartoftheanalysis.Themembranemodule
consists of a tube and shell arrangement of microporous
polypropylene hollow fibers. Sample flows along the inside of
The boldface numbers given in parentheses refer to a list of references at the
the fibers, while air is passed on the shell side-counterflow to
end of this standard.
thesampleflow.Theshellsidepressureisreducedbymeansof
avacuumpumpontheairoutlet.Thesampleisacidifiedbefore
TABLE 1 Blank Contribution and Inorganic Carbon (IC) Removal
Efficiency of Vacuum Degassing Unit introduction into the degasser to facilitate CO transport
A A through the hollow fibers. Sparging requires an inert vessel
Unit Number µg/L TOC µg/L IC IC Level with
Background Background
25 000 µg ⁄L Input
with a capacity of at least double the sample size with
1 3.2 8.2 55
provision for sparging with 50 to 100 mL/min of carbon free
2 3.2 22 61
gas. This procedure will remove essentially all IC in 2 to 10
3 2.4 8.0 105
min, depending on design.
4 4.2 13 89
5 2.8 13 30
7.2.2 Reaction—The sample flow is split after the addition
6 3.0 8.0 70
of reagents. Half of the flow passes to the delay coil while the
7 4.8 8.9 67
other half passes into the oxidation reactor. The effluent from
8 4.7 8.3 63
94.6 11 62
10 4.7 2.9 72
A
Values are the difference between before and after addition of the degasser to a
Instruments manufactured and marketed by Sievers Instruments, Inc., 2500
high purity (<5 µg/L) water stream.
Central Ave., Suite H1, Boulder, CO 80301, have been found satisfactory.
D5904 − 02 (2017)
bothstreamspassesoverindividualmembranesthatallowCO organic carbon contamination. Since halogens are potential
to pass through the membrane into prepurified water for interferences,useonlysulfuricorphosphoricacidforreagents.
detection. Sulfuric acid is prepared by diluting 336 mL of 95% reagent
7.2.3 Membrane—The membrane is a CO selective fluo- (sp gr 1.84) to 1 L with reagent water. Phosphoric acid is
ropolymer that is hydrophobic and non-porous. Refer to the prepared by diluting 410 mLof 85% reagent (sp gr 1.69) to 1
bibliography for additional details. Lwithwater.Certificationofreagentassayshouldbeavailable.
7.2.4 Detector—The CO that has passed through the mem- Reagentsinprepackagedcontainersfromtheinstrumentmanu-
brane into the purified water is measured by conductivity facturer have been found to be acceptable.
sensors. The temperature of the conductivity cell is also
8.5 Organic Carbon, Standard Solution (2000 mg/L)—
automatically monitored so the readings can be corrected for
Choose a water-soluble, stable reagent grade compound, such
changes in temperature.
as benzoic acid or anhydrous potassium hydrogen phthalate
7.2.5 Presentation of Results—The conductivity detector
(KHC H O ). Calculate the weight of compound required to
8 4 4
outputisrelatedtostoredcalibrationdataandthendisplayedas
make 1 L of organic carbon standard solution; for example,
parts per million, (ppm=milligrams of carbon per litre) or
KHC H O =0.471 g of carbon per gram, so 1 L of 2 g/L of
8 4 4
partsperbillion,(ppb=microgramsofcarbonperlitre).Values
standard requires 2/0.471, or 4.25, grams of KHP. Dissolve the
are given for TC, IC, and TOC by difference.
required amount of standard in some CO -free water in a 1-L
volumetric flask, add 1 mL of sulfuric acid, and dilute to
8. Reagents and Materials
volume.Dilutionsofthisstocksolutioncontaining20mg/Lare
8.1 Purity of Reagents—Reagent grade chemicals shall be
to be used to calibrate and test performance of the carbon
used in all tests. Unless otherwise indicated, it is intended that
analyzer.
all reagents conform to the specifications of the Committee on
AnalyticalReagentsoftheAmericanChemicalSociety, where
9. Sampling and Sample Preservation
such specifications are available. Other grades may be used,
9.1 CollectthesampleinaccordancewithGuideD1192and
provided it is first ascertained that the reagent is of sufficient
Practices D3370.
purity to permit its use without lessening the accuracy of the
determination.
9.2 To preserve samples for this analysis, store samples in
glass at 4°C. To aid preservation, acidify the samples to a pH
8.2 Purity of Water—Unless otherwise indicated, references
of 2. It should be noted that acidification will enhance loss of
towatershallbeunderstoodtomeanreagentwaterconforming
inorganiccarbon.Ifthepurgeableorganicfractionisimportant,
to Type I or Type II in Specification D1193. The indicated
fill the sample bottles to overflowing with a minimum of
specification does not actually specify inorganic carbon or
turbulence and cap them using a fluoropolymer-lined cap,
organiccarbonlevels.Theselevelscanaffecttheresultsofthis
without headspace.
test method, especially at progressively lower levels of the
carboncontentin
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D5904 − 02 (Reapproved 2017)
Standard Test Method for
Total Carbon, Inorganic Carbon, and Organic Carbon in
Water by Ultraviolet, Persulfate Oxidation, and Membrane
Conductivity Detection
This standard is issued under the fixed designation D5904; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.5 In addition to laboratory analyses, this test method may
be applied to on line monitoring.
1.1 This test method covers the determination of total
carbon (TC), inorganic carbon (IC), and total organic carbon 1.6 The values stated in SI units are to be regarded as
(TOC) in water in the range from 0.5 to 30 mg/L of carbon. standard. No other units of measurement are included in this
Higher levels may be determined by sample dilution. The test standard.
method utilizes ultraviolet-persulfate oxidation of organic
1.7 This standard does not purport to address all of the
carbon, coupled with a CO selective membrane to recover the
2 safety concerns, if any, associated with its use. It is the
CO into deionized water. The change in conductivity of the
responsibility of the user of this standard to establish appro-
deionized water is measured and related to carbon concentra-
priate safety, health, and environmental practices and deter-
tion in the oxidized sample. Inorganic carbon is determined in
mine the applicability of regulatory limitations prior to use.
a similar manner without the requirement for oxidation. In both
1.8 This international standard was developed in accor-
cases, the sample is acidified to facilitate CO recovery through
dance with internationally recognized principles on standard-
the membrane. The relationship between the conductivity
ization established in the Decision on Principles for the
measurement and carbon concentration is described by a set of
Development of International Standards, Guides and Recom-
chemometric equations for the chemical equilibrium of CO ,
mendations issued by the World Trade Organization Technical
− +
HCO , H , and the relationship between the ionic concentra-
Barriers to Trade (TBT) Committee.
tions and the conductivity. The chemometric model includes
the temperature dependence of the equilibrium constants and 2. Referenced Documents
the specific conductances.
2.1 ASTM Standards:
1.2 This test method has the advantage of a very high
D1129 Terminology Relating to Water
sensitivity detector that allows very low detection levels on
D1192 Guide for Equipment for Sampling Water and Steam
relatively small volumes of sample. Also, use of two measure-
in Closed Conduits (Withdrawn 2003)
ment channels allows determination of CO in the sample
D1193 Specification for Reagent Water
independently of organic carbon. Isolation of the conductivity
D2777 Practice for Determination of Precision and Bias of
detector from the sample by the CO selective membrane
Applicable Test Methods of Committee D19 on Water
results in a very stable calibration, with minimal interferences.
D3370 Practices for Sampling Water from Closed Conduits
D5810 Guide for Spiking into Aqueous Samples
1.3 This test method was used successfully with reagent
D5847 Practice for Writing Quality Control Specifications
water spiked with sodium bicarbonate and various organic
for Standard Test Methods for Water Analysis
materials. It is the user’s responsibility to ensure the validity of
this test method for waters of untested matrices.
3. Terminology
1.4 This test method is applicable only to carbonaceous
3.1 Definitions:
matter in the sample that can be introduced into the reaction
3.1.1 For definitions of terms used in this standard, refer to
zone. The injector opening size generally limits the maximum
Terminology D1129.
size of particles that can be introduced.
1 2
This test method is under the jurisdiction of ASTM Committee D19 on Water For referenced ASTM standards, visit the ASTM website, www.astm.org, or
and is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Organic Substances in Water. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Feb. 1, 2017. Published February 2017. Originally the ASTM website.
approved in 1996. Last previous edition approved in 2009 as D5904 – 02 (2009). The last approved version of this historical standard is referenced on
DOI: 10.1520/D5904-02R17. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5904 − 02 (2017)
3.2 Definitions of Terms Specific to This Standard: 4. Summary of Test Method
3.2.1 inorganic carbon (IC), n—carbon in the form of
4.1 Fundamentals—Carbon can occur in water as inorganic
carbon dioxide, carbonate ion, or bicarbonate ion.
and organic compounds. This test method can be used to make
independent measurements of IC and TC and can also deter-
3.2.2 potassium hydrogen phthalate (KHP), n—KHC H O .
8 4 4
mine TOC as the difference of TC and IC. If IC is high relative
3.2.3 refractory material, n—that which cannot be oxidized
to TOC it is desirable to use a vacuum degassing unit to reduce
completely under the test method conditions.
the IC concentration as part of the measurement. Alternatively,
3.2.4 total carbon (TC), n—the sum of IC and TOC.
the IC can be removed by acidifying and sparging the sample
prior to injection into the instrument.
3.2.5 total organic carbon (TOC), n—carbon in the form of
organic compounds. 4.2 The basic steps of this test method are:
FIG. 1 Schematic Diagram of TOC Analyzer System
D5904 − 02 (2017)
4.2.1 Removal of IC, if desired, by vacuum degassing; 6.2 Chloride ion above 250 mg/L tends to interfere with
4.2.2 Conversion of remaining inorganic carbon to CO by oxidative reaction mechanisms in this test method. Follow
action of acid in both channels and oxidation of total carbon to manufacturer’s instructions for dealing with this problem.
CO by action of acid-persulfate, aided by ultraviolet (UV) Other interferences have been investigated and found to be
radiation in the TC channel; minimal under most conditions. Refer to the references for
4.2.3 Detection of CO that is swept out of the reactors by more information.
the liquid stream over membranes that allow the specific
6.3 Note that error will be introduced when the method of
passage of CO to high purity water where change in conduc-
difference is used to derive a relatively small level from two
tivity is measured; and
large levels. For example, a ground water high in IC and low
4.2.4 Conversion of the conductivity detector signal to a
in TOC will give a poorer TOC value as (TC-IC) than by direct
display of carbon concentration in parts per million
measurement. In this case the vacuum degassing unit on the
(ppm = mg ⁄L) or parts per billion (ppb = µg ⁄L). The IC chan-
instrument should be used to reduce the concentration of IC
nel reading is subtracted from the TC channel to give a TOC
prior to measurement. Alternatively, the sample can be acidi-
reading. A diagram of suitable apparatus is given in Fig. 1.
fied and sparged prior to introduction into the instrument. Use
References (1-5) provide additional information on this test
of the vacuum degassing unit or sparging the sample may cause
method.
loss of volatile organic compounds, thus yielding a value lower
than the true TOC level.
5. Significance and Use
6.4 Use of the vacuum degassing unit or sparging the
5.1 This test method is used for determination of the carbon
sample may cause loss of volatile organic compounds, thus
content of water from a variety of natural, domestic, and
yielding a value lower than the true TOC level. At low TOC
industrial sources. In its most common form, this test method
levels, the degassing unit may introduce a measurable TOC and
is used to measure organic carbon as a means of monitoring
IC background. The user should characterize the background
organic pollutants in high purity and drinking water. These
and performance of the degassing module for their application.
measurements are also used in monitoring waste treatment
A removal efficiency of 97 % of the inlet IC is considered
processes.
satisfactory. Table 1 provides typical IC removal performance
5.2 The relationship of TOC to other water quality param-
and background levels of the vacuum degassing unit.
eters such as chemical oxygen demand (COD) and total oxygen
demand (TOD) is described in the literature (6).
7. Apparatus
6. Interferences and Limitations
7.1 Homogenizing Apparatus—A household blender is gen-
erally satisfactory for homogenizing immiscible phases in
6.1 The oxidation of dissolved carbon to CO is brought
water.
about at relatively low temperatures by the chemical action of
reactive species produced by UV-irradiated persulfate ions. Not
7.2 Apparatus for Carbon Determination—A typical instru-
all suspended or refractory material may be oxidized under
ment consists of reagent and sample introduction mechanism,
these conditions; analysts should take steps to determine what
reaction vessel, detector, control system, and a display. Fig. 1
recovery is being obtained. This may be done by several
shows a diagram of such an arrangement.
methods: by rerunning the sample under more vigorous reac-
7.2.1 Vacuum degassing requires the manufacturer’s mod-
tion conditions; by analyzing the sample by an alternative
ule that includes a vacuum pump and a hollow fiber mem-
method known to result in full recovery; or by spiking samples
brane assembly. Use of this vacuum degasser will remove
with known refractories and determining recovery.
essentially all IC as part of the analysis. The membrane module
consists of a tube and shell arrangement of microporous
polypropylene hollow fibers. Sample flows along the inside of
The boldface numbers given in parentheses refer to a list of references at the
the fibers, while air is passed on the shell side-counterflow to
end of this standard.
the sample flow. The shell side pressure is reduced by means of
a vacuum pump on the air outlet. The sample is acidified before
TABLE 1 Blank Contribution and Inorganic Carbon (IC) Removal
introduction into the degasser to facilitate CO transport
Efficiency of Vacuum Degassing Unit
A A
through the hollow fibers. Sparging requires an inert vessel
Unit Number µg/L TOC µg/L IC IC Level with
Background Background
25 000 µg ⁄L Input
with a capacity of at least double the sample size with
1 3.2 8.2 55
provision for sparging with 50 to 100 mL/min of carbon free
2 3.2 22 61
gas. This procedure will remove essentially all IC in 2 to 10
3 2.4 8.0 105
min, depending on design.
4 4.2 13 89
5 2.8 13 30
7.2.2 Reaction—The sample flow is split after the addition
6 3.0 8.0 70
of reagents. Half of the flow passes to the delay coil while the
7 4.8 8.9 67
other half passes into the oxidation reactor. The effluent from
8 4.7 8.3 63
9 4.6 11 62
10 4.7 2.9 72
A
Values are the difference between before and after addition of the degasser to a
high purity (<5 µg/L) water stream. Instruments manufactured and marketed by Sievers Instruments, Inc., 2500
Central Ave., Suite H1, Boulder, CO 80301, have been found satisfactory.
D5904 − 02 (2017)
both streams passes over individual membranes that allow CO organic carbon contamination. Since halogens are potential
to pass through the membrane into prepurified water for interferences, use only sulfuric or phosphoric acid for reagents.
detection. Sulfuric acid is prepared by diluting 336 mL of 95 % reagent
7.2.3 Membrane—The membrane is a CO selective fluo- (sp gr 1.84) to 1 L with reagent water. Phosphoric acid is
ropolymer that is hydrophobic and non-porous. Refer to the prepared by diluting 410 mL of 85 % reagent (sp gr 1.69) to 1
bibliography for additional details. L with water. Certification of reagent assay should be available.
7.2.4 Detector—The CO that has passed through the mem- Reagents in prepackaged containers from the instrument manu-
brane into the purified water is measured by conductivity facturer have been found to be acceptable.
sensors. The temperature of the conductivity cell is also
8.5 Organic Carbon, Standard Solution (2000 mg/L)—
automatically monitored so the readings can be corrected for
Choose a water-soluble, stable reagent grade compound, such
changes in temperature.
as benzoic acid or anhydrous potassium hydrogen phthalate
7.2.5 Presentation of Results—The conductivity detector
(KHC H O ). Calculate the weight of compound required to
8 4 4
output is related to stored calibration data and then displayed as
make 1 L of organic carbon standard solution; for example,
parts per million, (ppm = milligrams of carbon per litre) or
KHC H O = 0.471 g of carbon per gram, so 1 L of 2 g/L of
8 4 4
parts per billion, (ppb = micrograms of carbon per litre). Values
standard requires 2/0.471, or 4.25, grams of KHP. Dissolve the
are given for TC, IC, and TOC by difference.
required amount of standard in some CO -free water in a 1-L
volumetric flask, add 1 mL of sulfuric acid, and dilute to
8. Reagents and Materials
volume. Dilutions of this stock solution containing 20 mg/L are
8.1 Purity of Reagents—Reagent grade chemicals shall be
to be used to calibrate and test performance of the carbon
used in all tests. Unless otherwise indicated, it is intended that
analyzer.
all reagents conform to the specifications of the Committee on
Analytical Reagents of the American Chemical Society, where
9. Sampling and Sample Preservation
such specifications are available. Other grades may be used,
9.1 Collect the sample in accordance with Guide D1192 and
provided it is first ascertained that the reagent is of sufficient
Practices D3370.
purity to permit its use without lessening the accuracy of the
determination.
9.2 To preserve samples for this analysis, store samples in
glass at 4°C. To aid preservation, acidify the samples to a pH
8.2 Purity of Water—Unless otherwise indicated, references
of 2. It should be noted that acidification will enhance loss of
to water shall be understood to mean reagent water conforming
inorganic carbon. If the purgeable organic fraction is important,
to Type I or Type II in Specification D1193. The indicated
fill the sample bottles to overflowing with a minimum of
specification does not actually specify inorganic carbon or
turbulence and cap them usin
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D5904 − 02 (Reapproved 2009) D5904 − 02 (Reapproved 2017)
Standard Test Method for
Total Carbon, Inorganic Carbon, and Organic Carbon in
Water by Ultraviolet, Persulfate Oxidation, and Membrane
Conductivity Detection
This standard is issued under the fixed designation D5904; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the determination of total carbon (TC), inorganic carbon (IC), and total organic carbon (TOC) in
water in the range from 0.5 to 30 mg/L of carbon. Higher levels may be determined by sample dilution. The test method utilizes
ultraviolet-persulfate oxidation of organic carbon, coupled with a CO selective membrane to recover the CO into deionized water.
2 2
The change in conductivity of the deionized water is measured and related to carbon concentration in the oxidized sample.
Inorganic carbon is determined in a similar manner without the requirement for oxidation. In both cases, the sample is acidified
to facilitate CO recovery through the membrane. The relationship between the conductivity measurement and carbon
− +
concentration is described by a set of chemometric equations for the chemical equilibrium of CO , HCO , H , and the relationship
2 3
between the ionic concentrations and the conductivity. The chemometric model includes the temperature dependence of the
equilibrium constants and the specific conductances.
1.2 This test method has the advantage of a very high sensitivity detector that allows very low detection levels on relatively
small volumes of sample. Also, use of two measurement channels allows determination of CO in the sample independently of
organic carbon. Isolation of the conductivity detector from the sample by the CO selective membrane results in a very stable
calibration, with minimal interferences.
1.3 This test method was used successfully with reagent water spiked with sodium bicarbonate and various organic materials.
It is the user’s responsibility to ensure the validity of this test method for waters of untested matrices.
1.4 This test method is applicable only to carbonaceous matter in the sample that can be introduced into the reaction zone. The
injector opening size generally limits the maximum size of particles that can be introduced.
1.5 In addition to laboratory analyses, this test method may be applied to on line monitoring.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D1129 Terminology Relating to Water
D1192 Guide for Equipment for Sampling Water and Steam in Closed Conduits (Withdrawn 2003)
D1193 Specification for Reagent Water
D2777 Practice for Determination of Precision and Bias of Applicable Test Methods of Committee D19 on Water
D3370 Practices for Sampling Water from Closed Conduits
D5810 Guide for Spiking into Aqueous Samples
D5847 Practice for Writing Quality Control Specifications for Standard Test Methods for Water Analysis
This test method is under the jurisdiction of ASTM Committee D19 on Water and is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for
Organic Substances in Water.
Current edition approved Oct. 1, 2009Feb. 1, 2017. Published November 2009February 2017. Originally approved in 1996. Last previous edition approved in 20022007
as D5904–02. DOI: 10.1520/D5904-02R09. – 02 (2007). DOI: 10.1520/D5904-02R17.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
The last approved version of this historical standard is referenced on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5904 − 02 (2017)
3. Terminology
3.1 Definitions—Definitions: For definitions of terms used in this test method, refer to Terminology D1129.
3.1.1 For definitions of terms used in this standard, refer to Terminology D1129.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 inorganic carbon (IC)—(IC), n—carbon in the form of carbon dioxide, carbonate ion, or bicarbonate ion.
3.2.2 potassium hydrogen phthalate (KHP)—(KHP), n—KHC H O .
8 4 4
3.2.3 refractory material—material, n—that which cannot be oxidized completely under the test method conditions.
3.2.4 total carbon (TC)—(TC), n—the sum of IC and TOC.
3.2.5 total organic carbon (TOC)—(TOC), n—carbon in the form of organic compounds.
4. Summary of Test Method
4.1 Fundamentals—Carbon can occur in water as inorganic and organic compounds. This test method can be used to make
independent measurements of IC and TC and can also determine TOC as the difference of TC and IC. If IC is high relative to TOC
it is desirable to use a vacuum degassing unit to reduce the IC concentration as part of the measurement. Alternatively, the IC can
be removed by acidifying and sparging the sample prior to injection into the instrument.
4.2 The basic steps of this test method are:
4.2.1 Removal of IC, if desired, by vacuum degassing;
4.2.2 Conversion of remaining inorganic carbon to CO by action of acid in both channels and oxidation of total carbon to CO
2 2
by action of acid-persulfate, aided by ultraviolet (UV) radiation in the TC channel;
4.2.3 Detection of CO that is swept out of the reactors by the liquid stream over membranes that allow the specific passage
of CO to high purity water where change in conductivity is measured; and
4.2.4 Conversion of the conductivity detector signal to a display of carbon concentration in parts per million (ppm = mg ⁄L) or
parts per billion (ppb = μg ⁄L). The IC channel reading is subtracted from the TC channel to give a TOC reading. A diagram of
suitable apparatus is given in Fig. 1. References (1-5) provide additional information on this test method.
5. Significance and Use
5.1 This test method is used for determination of the carbon content of water from a variety of natural, domestic, and industrial
sources. In its most common form, this test method is used to measure organic carbon as a means of monitoring organic pollutants
in high purity and drinking water. These measurements are also used in monitoring waste treatment processes.
5.2 The relationship of TOC to other water quality parameters such as chemical oxygen demand (COD) and total oxygen
demand (TOD) is described in the literature.literature (6).
6. Interferences and Limitations
6.1 The oxidation of dissolved carbon to CO is brought about at relatively low temperatures by the chemical action of reactive
species produced by UV-irradiated persulfate ions. Not all suspended or refractory material may be oxidized under these
conditions; analysts should take steps to determine what recovery is being obtained. This may be done by several methods: by
rerunning the sample under more vigorous reaction conditions; by analyzing the sample by an alternative method known to result
in full recovery; or by spiking samples with known refractories and determining recovery.
6.2 Chloride ion above 250 mg/L tends to interfere with oxidative reaction mechanisms in this test method. Follow
manufacturer’s instructions for dealing with this problem. Other interferences have been investigated and found to be minimal
under most conditions. Refer to the references for more information.
6.3 Note that error will be introduced when the method of difference is used to derive a relatively small level from two large
levels. For example, a ground water high in IC and low in TOC will give a poorer TOC value as (TC-IC) than by direct
measurement. In this case the vacuum degassing unit on the instrument should be used to reduce the concentration of IC prior to
measurement. Alternatively, the sample can be acidified and sparged prior to introduction into the instrument. Use of the vacuum
degassing unit or sparging the sample may cause loss of volatile organic compounds, thus yielding a value lower than the true TOC
level.
6.4 Use of the vacuum degassing unit or sparging the sample may cause loss of volatile organic compounds, thus yielding a
value lower than the true TOC level. At low TOC levels, the degassing unit may introduce a measurable TOC and IC background.
The user should characterize the background and performance of the degassing module for their application. A removal efficiency
of 97 % of the inlet IC is considered satisfactory. Table 1 provides typical IC removal performance and background levels of the
vacuum degassing unit.
The boldface numbers given in parentheses refer to a list of references at the end of this standard.
D5904 − 02 (2017)
FIG. 1 Schematic Diagram of TOC Analyzer System
TABLE 1 Blank Contribution and Inorganic Carbon (IC) Removal
Efficiency of Vacuum Degassing Unit
A A
Unit Number μg/L TOC μg/L IC IC Level with
Background Background
25 000μ g/L25 000
μg ⁄L Input
1 3.2 8.2 55
2 3.2 22 61
3 2.4 8.0 105
4 4.2 13 89
5 2.8 13 30
6 3.0 8.0 70
7 4.8 8.9 67
8 4.7 8.3 63
9 4.6 11 62
10 4.7 2.9 72
A
Values are the difference between before and after addition of the degasser to a
high purity (<5 μg/L) water stream.
D5904 − 02 (2017)
7. Apparatus
7.1 Homogenizing Apparatus—A household blender is generally satisfactory for homogenizing immiscible phases in water.
7.2 Apparatus for Carbon Determination—A typical instrument consists of reagent and sample introduction mechanism,
reaction vessel, detector, control system, and a display. Fig. 1 shows a diagram of such an arrangement.
7.2.1 Vacuum degassing requires the manufacturer’s module that includes a vacuum pump and a hollow fiber membrane
assembly. Use of this vacuum degasser will remove essentially all IC as part of the analysis. The membrane module consists of
a tube and shell arrangement of microporous polypropylene hollow fibers. Sample flows along the inside of the fibers, while air
is passed on the shell side-counterflow to the sample flow. The shell side pressure is reduced by means of a vacuum pump on the
air outlet. The sample is acidified before introduction into the degasser to facilitate CO transport through the hollow fibers.
Sparging requires an inert vessel with a capacity of at least double the sample size with provision for sparging with 50 to 100
mL/min of carbon free gas. This procedure will remove essentially all IC in 2 to 10 min, depending on design.
7.2.2 Reaction—The sample flow is split after the addition of reagents. Half of the flow passes to the delay coil while the other
half passes into the oxidation reactor. The effluent from both streams passes over individual membranes that allow CO to pass
through the membrane into prepurified water for detection.
7.2.3 Membrane—The membrane is a CO selective fluoropolymer that is hydrophobic and non-porous. Refer to the
bibliography for additional details.
7.2.4 Detector—The CO that has passed through the membrane into the purified water is measured by conductivity sensors.
The temperature of the conductivity cell is also automatically monitored so the readings can be corrected for changes in
temperature.
7.2.5 Presentation of Results—The conductivity detector output is related to stored calibration data and then displayed as parts
per million, (ppm = milligrams of carbon per litre) or parts per billion, (ppb = micrograms of carbon per litre). Values are given
for TC, IC, and TOC by difference.
8. Reagents and Materials
8.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such
specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficient purity to permit
its use without lessening the accuracy of the determination.
8.2 Purity of Water—Unless otherwise indicated, references to water shall be understood to mean reagent water conforming to
Type I or Type II in Specification D1193. The indicated specification does not actually specify inorganic carbon or organic carbon
levels. These levels can affect the results of this test method, especially at progressively lower levels of the carbon content in the
samples to be measured. Where inorganic carbon in reagent water is significant, CO -free water may be prepared from reagent
water by acidifying to pH 2, then sparging with fritted-glass sparger using CO -free gas (time will depend on volume and gas flow
rate, and should be determined by test). The carbon contribution of the reagent water should be determined and its effect allowed
for in preparation of standards and other solutions. CO -free water should be protected from atmospheric contamination. Glass
containers are required for storage of water and standard solutions.
8.3 Persulfate Reagent (15 % w/v)—Prepare ammonium persulfate to a concentration of 15 % w/v by dissolving 15 g of
ammonium peroxydisulfate in water and diluting to 100 mL. Verify that it contains less than 2000 μg/L organic carbon
contamination. Certification of reagent assay should be available. Reagents in prepackaged containers from the instrument
manufacturer have been found to be acceptable.
8.4 Acid Reagent (6M)—Prepare acid solution to a concentration of 6M and verify that it contains less than 600 μg/L organic
carbon contamination. Since halogens are potential interferences, use only sulfuric or phosphoric acid for reagents. Sulfuric acid
is prepared by diluting 336 mL of 95 % reagent (sp gr 1.84) to 1 L with reagent water. Phosphoric acid is prepared by diluting
410 mL of 85 % reagent (sp gr 1.69) to 1 L with water. Certification of reagent assay should be available. Reagents in prepackaged
c
...











Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.