ASTM D6208-97
(Test Method)Standard Test Method for Repassivation Potential of Aluminum and Its Alloys by Galvanostatic Measurement
Standard Test Method for Repassivation Potential of Aluminum and Its Alloys by Galvanostatic Measurement
SCOPE
1.1 A procedure to determine the repassivation potential of aluminum alloy 3003-H14 (UNS A93003) (1) as a measure of relative susceptibility to pitting corrosion by conducting a galvanostatic polarization is described. A procedure that can be used to check experimental technique and instrumentation is described, as well.
1.2 The test method serves as a guide for similar measurement on other aluminum alloys and metals (2-5).
1.3 The values stated in SI units are to be regarded as the standard. Values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 6208 – 97 An American National Standard
Standard Test Method for
Repassivation Potential of Aluminum and Its Alloys by
Galvanostatic Measurement
This standard is issued under the fixed designation D 6208; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope with an explanation provided where applicable. Terms used in
this test method can be found in Practice G 3 and Terminology
1.1 A procedure to determine the repassivation potential of
G 15.
aluminum alloy 3003-H14 (UNS A93003) (1) as a measure of
3.2 Symbols:
relative susceptibility to pitting corrosion by conducting a
3.2.1 E —break potential, potential at which the passive
B
galvanostatic polarization is described. A procedure that can be
aluminum oxide layer breaks down.
used to check experimental technique and instrumentation is
3.2.2 E —protection potential as measured in this galvano-
G
described, as well.
static method, potential at which oxide layer repassivates.
1.2 The test method serves as a guide for similar measure-
3.2.3 J—current density, in A/m
ment on other aluminum alloys and metals (2-5).
1.3 The values stated in SI units are to be regarded as the
4. Summary of Test Method
standard. Values given in parentheses are for information only.
4.1 The test method described is an adaptation of the
1.4 This standard does not purport to address all of the
method described in FORD Motor Company standards (6).
safety concerns, if any, associated with its use. It is the
4.2 An aluminum alloy specimen is polarized at fixed
responsibility of the user of this standard to establish appro-
current density for 20 min. in a solution of coolant and
priate safety and health practices and determine the applica-
corrosive water containing chloride. The potential as a function
bility of regulatory limitations prior to use.
of time is recorded.
2. Referenced Documents 4.3 The maximum potential, E reached upon polarization
B
is determined, as is the minimum potential following the
2.1 ASTM Standards:
maximum potential, E .
G
D 1193 Specification for Reagent Waters
4.4 Visual examination of the specimen may be made using
D 3585 Specification ASTM Reference Fluid for Coolants
Guide G 46 as a guide after disassembly and rinsing.
Tests
G 3 Practice for Conventions Applicable to Electrochemical
5. Significance and Use
measurements in Corrosion Testing
5.1 This test method is designed to measure the relative
G 15 Terminology Relating to Corrosion and Corrosion
effectiveness of inhibitors to mitigate pitting corrosion of
Testing
aluminum and its alloys, in particular AA3003-H14, rapidly
G 16 Guide for Applying Statistics to Analysis of Corrosion
and reproducibly. The measurements are not intended to
Data
correlate quantitatively with other test method values or with
G 46 Guide for Examination and Evaluation of Pitting
5 susceptibility to localized corrosion of aluminum observed in
Corrosion
service. Qualitative correlation of the measurements and sus-
G 107 Guide for Formats for Collection and Compilation of
ceptibility in service has been established (1).
Corrosion Data for Metals for Computerized Database
5 5.2 The maximum potential reached upon initial polariza-
Input
tion, E is a measure of the resistance to breakdown of the
B,
3. Terminology aluminum oxide film. Lower susceptibility to initiation of
pitting corrosion is indicated by a more noble potential (See
3.1 Definitions: An attempt to avoid terminology is made,
Practice G 3 and Terminology G 15.) This potential, as mea-
sured in this test method, is not very sensitive to the inhibitors
This test method is under the jurisdiction of ASTM Committee D15 on Engine
present.
Coolants and is the direct responsibility of Subcommittee D15.06 on Glassware
Performance Tests. 5.3 The minimum potential, E following the maximum
G,
Current edition approved Dec. 10, 1997. Published July 1998.
potential is a measure of the protection against continued
The boldface numbers in parentheses refer to the list of references at the end of
pitting corrosion by the inhibitors. Again, a more noble
this standard.
potential indicates better protection. This potential is sensitive
Annual Book of ASTM Standards, Vol 11.01.
Annual Book of ASTM Standards, Vol 15.05.
to the inhibitors present.
Annual Book of ASTM Standards, Vol 03.02.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
D 6208
5.4 Visual examination of the specimens can provide infor- ration is desirable. Prior to testing, a coupon is allowed to cool
mation about subleties of the pitting and inhibition mecha- to room temperature. Then it is clamped to the bottom of the
nisms. Number of pits, pit depth, amount of deposit, and O-ring joint using the matching O-ring (viton or silicone
surface discoloration are some examples of recordable obser- rubber) and clamp. The clamping screw may be tightened to
vations, which can assist evaluation of inhibitor effectiveness. finger tightness, if desired. Excessive tightening must be
5.5 The presence of chloride in the test solution is critical to avoided. This gives an area of 8.72 cm aluminum exposed to
observation of pitting corrosion. Also, a coolant/corrosive the solution.
water solution in which gas bubbles evolve spontaneously on 6.4.2 Auxiliary Electrode (AE)—Ultrafine grade graphite
the aluminum (indicating general corrosion) is unlikely to have rod, 6-8 mm (1/4 in.) in diameter and at least 20 cm (8 in.)
a significant amount of observable pitting corrosion. long. Avoid coarse grades as they can adsorb inhibitors.
6.4.3 Reference Electrode (RE)—The reference electrode
6. Apparatus
can be of any convenient type, for example saturated calomel
(Hg/HgCl) or silver chloride (Ag/AgCl). The electrode must be
6.1 General Description—The apparatus for the electro-
in good working order and stable in the solution to be
chemical test consists of a cell, current supply, recorder, and
measured. The reference electrode is placed in Luggin probe to
three electrodes. Fig. 1 is a generalized schematic of the
avoid solution impedance bias. Appendix X2 contains two
arrangement. More specific requirements for each component
are given below. suggestions for easily constructed Luggin probes.
6.5 Timer—Timer with 1 s resolution out to 30 min.
6.2 Cell—The cell consists of a No.25 O-ring borosilicate
glass joint held vertically using standard laboratory clamps and
7. Preparation of Apparatus
ring stand. The working electrode will be clamped to the
7.1 Assembly—Prior to running tests, assemble the cell and
bottom using the matching O-ring clamp and viton or silicone
electrodes, using an unprepared Al specimen as the “working”
rubber gasket.
electrode using appropriate clamping. The auxiliary electrode
6.3 Current Supply and Recorder—A constant current sup-
is positioned so that the tip is from 5 to 10 mm from the
ply capable of generating 872 μA continuously is required. The
working electrode surface. The Luggin probe is positioned so
recorder must have a high input impedance (> 10 Ohms), be
that the tip is from 1 to 3 mm from the working electrode
capable of recording potentials of 62 V with mV accuracy, and
surface. It is most convenient if the clamping arrangement is
have a low gain. These capabilities are typical of commercial
such that this electrode configuration is maintained easily. The
potentiostat/galvanostat instruments connected to either a strip
cell is then removed and Al specimen unclamped.
chart recorder or computer, for experimental control and data
acquisition. The schematic in Fig. 1 shows connections using a
8. Procedure
current supply and mV strip chart recorder, and Fig. X2.1
8.1 A corrosive water containing chloride, sulfate, and
shows a schematic for using a computer and potentiostat/
bicarbonate is prepared by dissolving the following amounts of
galvanostat.
anhydrous salts in distilled or deionized water, ASTM Type II
6.4 Electrodes:
(see Specification D 1193):
6.4.1 Working Electrode (WE)—The working electrode,
Sodium sulfate 592 mg
aluminum test coupon, is cut as 51 3 51 mm (2 in. 3 2 in. )
Sodium chloride 660 mg
squares from aluminum sheet 2 to 6 mm (1/16 in. to 1/4 in.)
Sodium bicarbonate 552 mg
thick. The standard material is AA3003-H14 (UNS A93003),
The solution is made up to a total weight of 1 kg with
used to develop the precision and bias statements. The coupon
distilled or deionized water at 20°C. A 4-kg batch size is
is rinsed thoroughly (both sides) with methanol and placed in
convenient if many tests are to be run, multiply amounts above
a low temperature drying oven. No additional surface prepa-
by four. This will give a solution, which is 400 ppm in chloride,
sulfate, and bicarbonate.
8.2 Rinse cell, O-ring, Luggin probe (inside and out),
auxilliary electrode, and reference electrode thoroughly with
Type II water.
8.3 Prepare the aluminum specimen as the working elec-
trode (see 5.4.2). Clamp to cell, using O-ring, and set to one
side.
8.4 Prepare the test solution as 25 vol % of the coolant to be
tested, 25 vol % of the corrosive water from 6.1, and the
remainder deionized or distilled water. The amount to be made
depends on one’s exact cell configuration. Sufficient test
solution is required to fill the cell (about 50 mLs) and the
Luggin probe assembly. For the configurations of Luggin probe
given in Appendix X2, 160 mLs is more than sufficient.
8.5 Fill the Luggin probe with test solution sufficient to
cover the tip of reference electrode when inserted. Insert
FIG. 1 Generalized Experimental Set-up reference electrode. Gently tap Luggin to remove any bubbles
D 6208
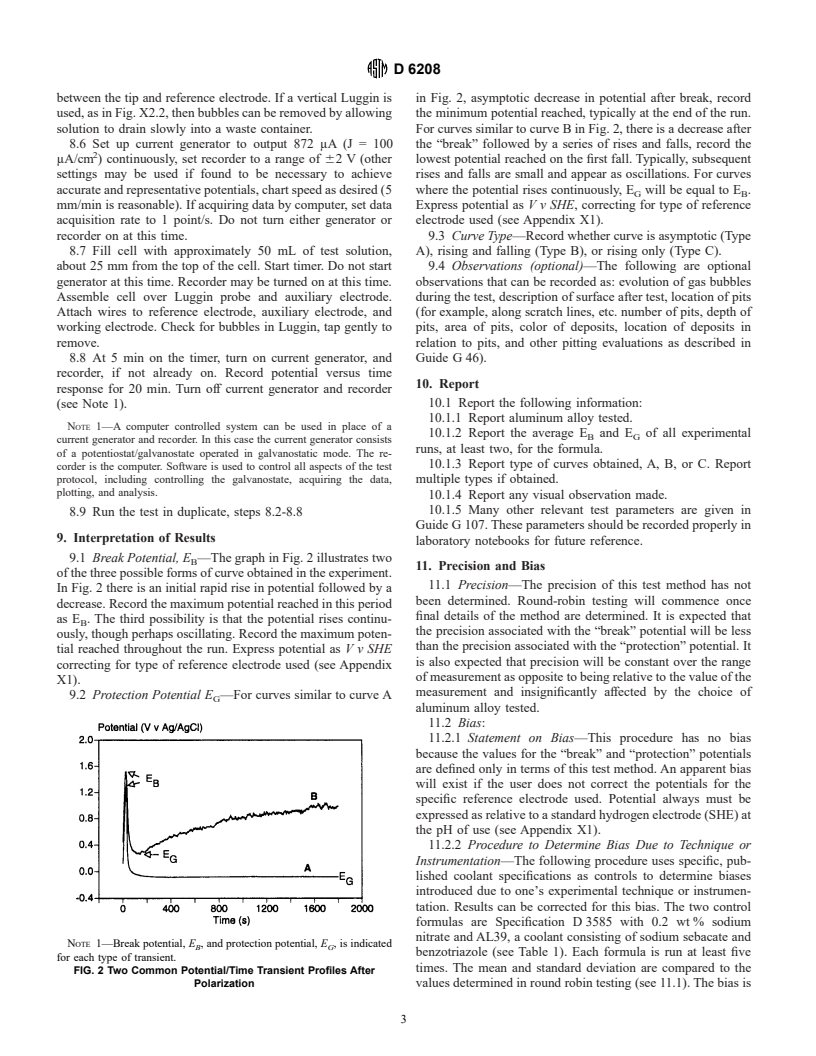
between the tip and reference electrode. If a vertical Luggin is in Fig. 2, asymptotic decrease in potential after break, record
used, as in Fig. X2.2, then bubbles can be removed by allowing the minimum potential reached, typically at the end of the run.
solution to drain slowly into a waste container. For curves similar to curve B in Fig. 2, there is a decrease after
8.6 Set up current generator to output 872 μA (J = 100 the “break” followed by a series of rises and falls, record the
μA/cm ) continuously, set recorder to a range of 62 V (other lowest potential reached on the first fall. Typically, subsequent
settings may be used if found to be necessary to achieve rises and falls are small and appear as oscillations. For curves
accurate and representative potentials, chart speed as desired (5 where the potential rises continuously, E will be equal to E .
G B
mm/min is reasonable). If acquiring data by computer, set data Express potential as V v SHE, correcting for type of reference
acquisition rate to 1 point/s. Do not turn either generator or electrode used (see Appendix X1).
recorder on at this time. 9.3 Curve Type—Record whether curve is asymptotic (Type
8.7 Fill cell with approximately 50 mL of test solution, A), rising and falling (Type B), or rising only (Type C).
about 25 mm from the top of the cell. Start timer. Do not start 9.4 Observations (optional)—The following are optional
generator at this time. Recorder may be turned on at this time. observations that can be recorded as: evolution of gas bubbles
Assemble cell over Luggin probe and auxiliary electrode. during the test, description of surface after test, location of pits
Attach wires to reference electrode, auxiliary electrode, and (for example, along scratch lines, etc. number of pits, depth of
working electrode. Check for bubbles in Luggin, tap gently to pits, area of pits, color of deposits, location of deposits in
remove. relation to pits, and other pitting evaluations as described in
8.8 At 5 min on the timer, turn on current generator, and Guide G 46).
recorder, if not already on. Record potential versus time
10. Report
response for 20 min. Turn off current generator and recorder
(see Note 1). 10.1 Report the following information:
10.1.1 Report aluminum alloy tested.
NOTE 1—A computer controlled system can be used in place of a
10.1.2 Report the average E and E of all experimental
B G
current generator and recorder. In this case the current generator consists
runs, at least two, for the formula.
of a potentiostat/galvanostate operated in galvanostatic mode. The re-
10.1.3 Report type of curves obtained, A, B, or C. Report
corder is the computer. Software is used to control all aspects of the test
protocol, including controlling the galvanostate, acquiring the data, multiple types if obtained.
plotting, and analysis.
10.1.4 Report any visual observation made.
10.1.5 Many other relevant test parameters are given in
8.9 Run the test in duplicate, steps 8.2-8.8
Guide G 107. These parameters should be recorded properly in
9. Interpretation of Results
laboratory notebooks for future reference.
9.1 Break Potential, E —The graph in Fig. 2 illustrates two
B
11. Precision and Bias
of the three possible forms of curve obtained in the experiment.
11.1 Precision—The precision of this test method has not
In Fig. 2 there is an initial rapid rise in potential followed by a
been determined. Round-robin testing will commence once
decrease. Record the maximum potential reached in this period
final details of the method are determined. It is expected that
as E . The third possibility is that the potential rises continu-
B
the precision associated with the “break” potential will be less
ously, though perhaps oscillating. Record the maximum poten-
than the precision associated with the “protection” potential. It
tial reached throughout the run. Express potential as V v SHE
is also expected that precision will be constant over the range
correcting for type of reference electrode used (see Appendix
of measurement as opposite to being relative to the value of the
X1).
measurement and insignificantly affected by the choice of
9.2 Protection Potential E —For curves similar to curve A
G
aluminum alloy tested.
11.2 Bias:
11.2.1 Statement on Bias—This procedure has no bias
because the values for the “break” and “protection” potentials
are defined only in terms of this test method. An apparent bias
will exist if the user does not correct the potentials for the
specific reference electrode used. Potential always must be
expressed as relative to a standard hydrogen electrode (SHE) at
the pH of use (see Appendix X1).
11.2.2 Procedure to Determine Bias Due to Technique or
Instrumentation—The following procedure uses specific, pub-
lished coolant specifications as controls to determine biases
introduced due to one’s experim
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.