ASTM D3268-91(2018)
(Test Method)Standard Test Method for Separation and Collection of Particulate and Gaseous Fluorides in the Atmosphere (Sodium Bicarbonate-Coated Glass Tube and Particulate Filter Method)
Standard Test Method for Separation and Collection of Particulate and Gaseous Fluorides in the Atmosphere (Sodium Bicarbonate-Coated Glass Tube and Particulate Filter Method)
SIGNIFICANCE AND USE
5.1 The sodium bicarbonate coated tube filter method provides a means of separating and collecting atmospheric gaseous fluoride and particulate fluoride samples.
5.2 Since the samples are collected on the dry tube and filter, the fluoride may be eluted with a small volume of eluant (see Section 10 for specific instructions on fluoride elution). Elution into a small volume and the sensitivity of the analytical methods employed allow the analysis of the collected fluoride to fractional parts of a microgram per cubic metre on samples taken for a 12-h period.
SCOPE
1.1 The sodium bicarbonate-coated glass tube and membrane filter method provides a means for the separation and collection of gaseous atmospheric forms of fluoride reactive with sodium bicarbonate and particulate forms of fluoride which are collected by a filter. The test method is applicable to 12-h sampling periods, collecting 1 to 500 μg of gaseous fluoride at a 15 L/min (0.5 ft3/min) sampling rate or about 0.1 to 50 μg/m3. The length of the sampling period can therefore be adjusted so that the amount of fluoride collected will fall within this range. The actual lower limit of the test method will depend upon the sensitivity of the analytical method employed and the quality of reagents used in tube preparation and analysis. It is recommended that the lower limit of detection should be considered as two times the standard deviation of the monthly arithmetic mean blank value. Any values greater than the blank by less than this amount should be reported as “blank value.”
1.2 The values stated in SI units are to be regarded as standard. The values given in parentheses are mathematical conversions to inch-pound units that are provided for information only and are not considered standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D3268 − 91 (Reapproved 2018)
Standard Test Method for
Separation and Collection of Particulate and Gaseous
Fluorides in the Atmosphere (Sodium Bicarbonate-Coated
Glass Tube and Particulate Filter Method)
This standard is issued under the fixed designation D3268; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope 2. Referenced Documents
1.1 The sodium bicarbonate-coated glass tube and mem- 2.1 ASTM Standards:
brane filter method provides a means for the separation and D1193 Specification for Reagent Water
collection of gaseous atmospheric forms of fluoride reactive D1356 Terminology Relating to Sampling and Analysis of
with sodium bicarbonate and particulate forms of fluoride Atmospheres
which are collected by a filter. The test method is applicable to D1357 Practice for Planning the Sampling of the Ambient
12-h sampling periods, collecting 1 to 500 µg of gaseous Atmosphere
fluoride at a 15 L/min (0.5 ft /min) sampling rate or about 0.1 D3266 Test Method for Automated Separation and Collec-
to50µg/m .Thelengthofthesamplingperiodcanthereforebe tion of Particulate and Acidic Gaseous Fluoride in the
adjustedsothattheamountoffluoridecollectedwillfallwithin Atmosphere (Double Paper Tape Sampler Method)
this range. The actual lower limit of the test method will D3267 Test Method for Separation and Collection of Par-
depend upon the sensitivity of the analytical method employed ticulate and Water-Soluble Gaseous Fluorides in the At-
and the quality of reagents used in tube preparation and mosphere (Filter and Impinger Method)
analysis. It is recommended that the lower limit of detection D3269 Test Methods for Analysis for Fluoride Content of
shouldbeconsideredastwotimesthestandarddeviationofthe the Atmosphere and Plant Tissues (Manual Procedures)
monthly arithmetic mean blank value.Any values greater than (Withdrawn 2010)
the blank by less than this amount should be reported as “blank D3270 Test Methods for Analysis for Fluoride Content of
value.” the Atmosphere and Plant Tissues (Semiautomated
Method)
1.2 The values stated in SI units are to be regarded as
standard. The values given in parentheses are mathematical
3. Terminology
conversions to inch-pound units that are provided for informa-
3.1 Definitions—For definitions of terms used in this test
tion only and are not considered standard.
method, refer to Terminology D1356.
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
4. Summary of Test Method
responsibility of the user of this standard to establish appro-
4.1 Gaseous fluorides are removed from the air stream by
priate safety, health, and environmental practices and deter-
reaction with sodium bicarbonate coated on the inside wall of
mine the applicability of regulatory limitations prior to use.
a borosilicate glass tube (Note 1). Particulate fluorides are
1.4 This international standard was developed in accor-
collected on a filter following the tube. The fluoride collected
dance with internationally recognized principles on standard-
by the tube is eluted with water or buffer and analyzed for
ization established in the Decision on Principles for the
fluoride. The particulate matter collected by the filter is eluted
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee.
1 2
This test method is under the jurisdiction of ASTM Committee D22 on Air For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Quality and is the direct responsibility of Subcommittee D22.03 on Ambient contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Atmospheres and Source Emissions. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved April 15, 2018. Published May 2018. Originally the ASTM website.
approved in 1973. Last previous edition approved in 2011 as D3268 – 91 (2011). The last approved version of this historical standard is referenced on
DOI: 10.1520/D3268-91R18. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3268 − 91 (2018)
FIG. 1 Sodium Bicarbonate-Coated Glass Tube Illustrating Simple Heating Device
with acid and analyzed for fluoride (1-4). The results are 5.2 Since the samples are collected on the dry tube and
reported as µg/m of gaseous or particulate in air at 25°C filter, the fluoride may be eluted with a small volume of eluant
(77°F) and 101.3 kPa (29.92 in. Hg). (see Section 10 for specific instructions on fluoride elution).
Elutionintoasmallvolumeandthesensitivityoftheanalytical
NOTE 1—Some particulate matter will collect on the wall of the sample
methods employed allow the analysis of the collected fluoride
tube. If this loss is to be evaluated, use 7test methods such asTest Method
to fractional parts of a microgram per cubic metre on samples
D3266orTestMethodD3267forcomparisonsincethefilterforcollecting
particulate precedes the absorbers for gases Mandl and Weinstein (2)
taken for a 12-h period.
provide some information relative to potential loss of particulate matter.
6. Interferences
5. Significance and Use
6.1 Significant amounts of acid aerosols or gases might
5.1 The sodium bicarbonate coated tube filter method pro-
neutralize or acidify the bicarbonate coating and prevent
vides a means of separating and collecting atmospheric gas-
quantitative uptake of gaseous fluoride from the atmosphere. If
eous fluoride and particulate fluoride samples.
this potential interference needs to be evaluated, the alkalinity
of the water extract may provide relevant information.
4 6.2 The presence of large amounts of aluminum or certain
The boldface numbers in parentheses refer to references at the end of this test
method. other metals or phosphates can interfere with subsequent
D3268 − 91 (2018)
analyses of the tubes or filters by calorimetric or electrometric 7.3 Air Sampling System:
methods. This is a problem inherent with any collection
7.3.1 The tube and filter are followed by an air sampling
method for fluoride.
system which is capable of sampling at a rate of 15 L/min (0.5
ft /min) and measuring the total air sampled on a time rate
7. Apparatus
basis or with a totalizing meter. See Test Method D3267 for
7.1 Glass Tubing—1200-mm (4-ft) lengths of 7-mm inside
sampling equipment, and the configuration and calibration.
diameter borosilicate glass tubing, coated with sodium
7.3.2 The system shall be equipped so that pressure and
bicarbonate, in accordance with the requirements outlined in
temperature of the gas at the point of metering also are known
7.6.
for correcting sample volumes to standard conditions of 101.3
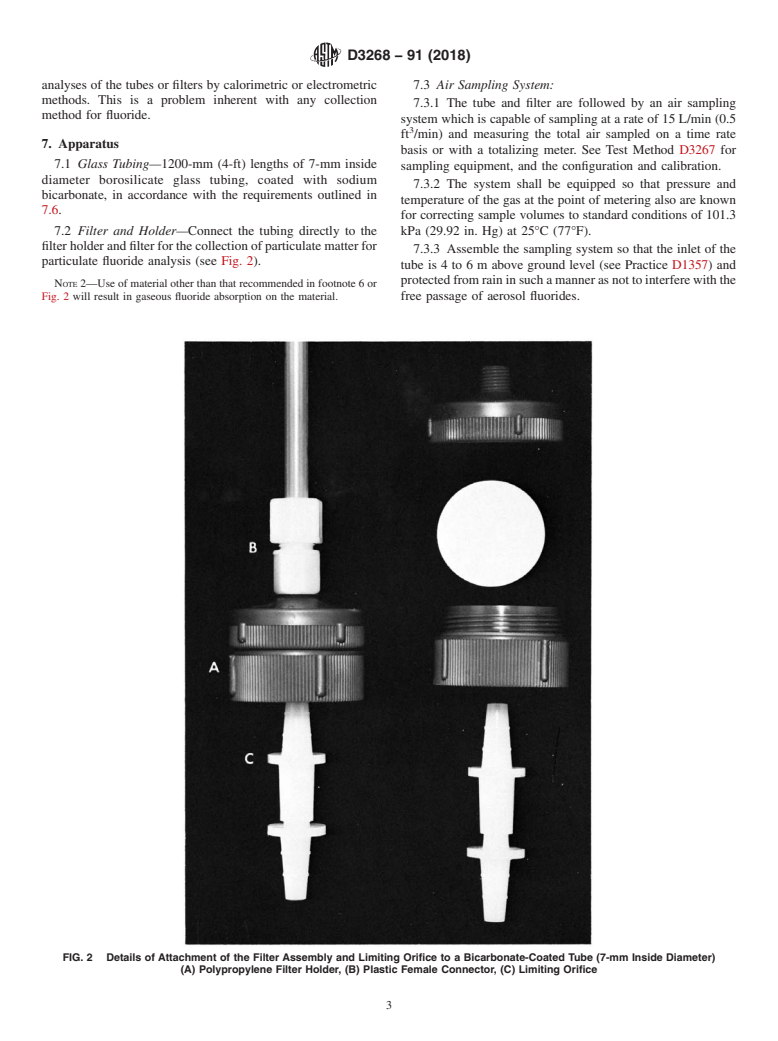
7.2 Filter and Holder—Connect the tubing directly to the kPa (29.92 in. Hg) at 25°C (77°F).
filterholderandfilterforthecollectionofparticulatematterfor
7.3.3 Assemble the sampling system so that the inlet of the
particulate fluoride analysis (see Fig. 2).
tube is 4 to 6 m above ground level (see Practice D1357) and
protectedfromraininsuchamannerasnottointerferewiththe
NOTE 2—Use of material other than that recommended in footnote 6 or
Fig. 2 will result in gaseous fluoride absorption on the material. free passage of aerosol fluorides.
FIG. 2 Details of Attachment of the Filter Assembly and Limiting Orifice to a Bicarbonate-Coated Tube (7-mm Inside Diameter)
(A) Polypropylene Filter Holder, (B) Plastic Female Connector, (C) Limiting Orifice
D3268 − 91 (2018)
7.4 Light Bulb or Cone Heater, 30-W, installed to heat the ina1000-mLvolumetricflask.Swirltomix,cool,anddiluteto
gases to a temperature where condensation will not occur. 1000 mL with reagent water. Mix thoroughly.
7.5 Configuration of Sampling Equipment—Fig. 1 is a 8.7 Sodium Hydroxide Solution (5 N)—Dissolve 200 g of
sketch of the sampling system. Other systems that meet the NaOH in 250 mL of reagent water in a 1000-mL volumetric
requirements outlined, are also satisfactory. flask. Swirl to mix, cool, and dilute to 1000 mL with reagent
water. Mix thoroughly.
7.6 Criteria for Coating of the Borosilicate Tubes:
7.6.1 Thecoatingshallbevisibleuniformcoatingonthefull 8.8 Sulfuric Acid (1.0 N)—Add 28 mL of concentrated
length of the tube. H SO (sp gr 1.84) to 250 mL of reagent water in a 1000-mL
2 4
7.6.2 The coating shall not contain any large crystals or volumetric flask. Swirl to mix, cool, and dilute to 1000 mL
heavy local deposits which could flake off and be collected with reagent water. Mix thoroughly.
with the aerosol fluorides.
8.9 Total Ionic Strength Adjustment Buffer (TISAB)—Add
7.6.3 The total coating shall contain less than 1 µg of
57 mL of glacial acetic acid, 58 g of sodium chloride (NaCl)
fluoride when analyzed without exposure, including all the
and 4.0 g of CDTA [(1,2-cyclohexylenedinitrilo)tetraacetic
reagentsusedintheprocedure.Thisisthereagentblankforthe
acid] to 500 mL of distilled water. Stir and add 5 N NaOH
procedure.
solution (8.7) slowly until pH is between 5.0 and 5.5. Cool and
7.6.4 Preparedtubesshallbesealeduntiltimeofuse.Serum
dilute to 1 L.
tube caps that have been thoroughly rinsed with reagent
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.