ASTM F3437-23
(Test Method)Standard Test Methods for Metallic Bone Plates Used in Small Bone Fracture Fixation
Standard Test Methods for Metallic Bone Plates Used in Small Bone Fracture Fixation
SIGNIFICANCE AND USE
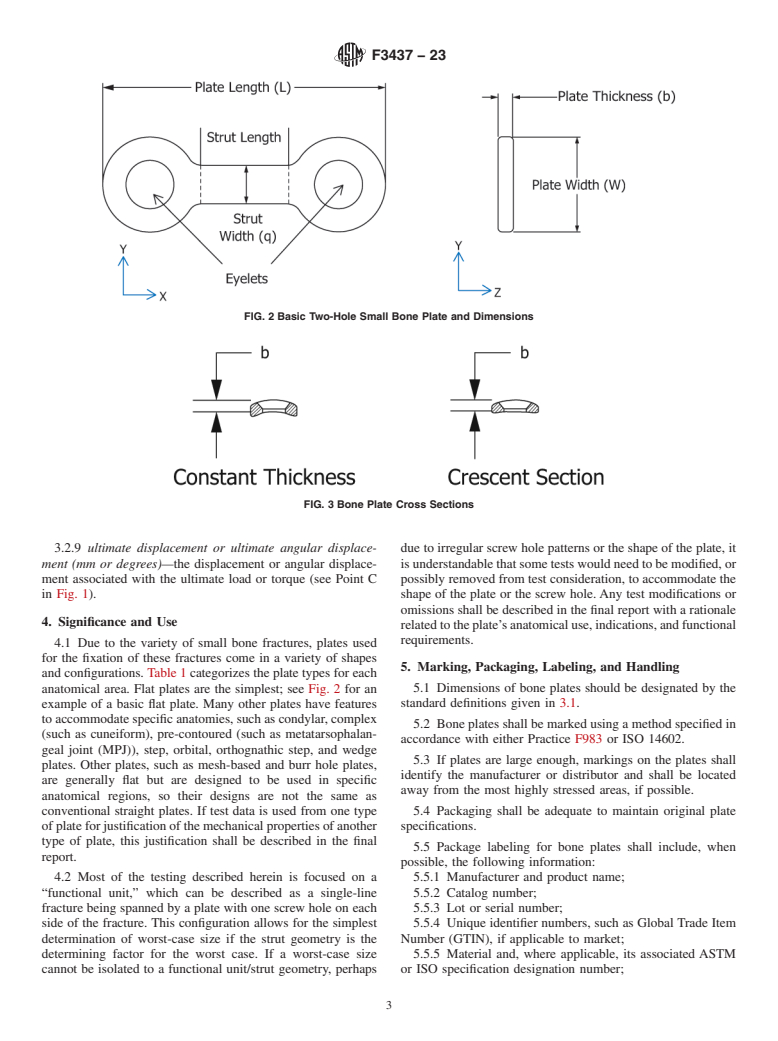
4.1 Due to the variety of small bone fractures, plates used for the fixation of these fractures come in a variety of shapes and configurations. Table 1 categorizes the plate types for each anatomical area. Flat plates are the simplest; see Fig. 2 for an example of a basic flat plate. Many other plates have features to accommodate specific anatomies, such as condylar, complex (such as cuneiform), pre-contoured (such as metatarsophalangeal joint (MPJ)), step, orbital, orthognathic step, and wedge plates. Other plates, such as mesh-based and burr hole plates, are generally flat but are designed to be used in specific anatomical regions, so their designs are not the same as conventional straight plates. If test data is used from one type of plate for justification of the mechanical properties of another type of plate, this justification shall be described in the final report.
4.2 Most of the testing described herein is focused on a “functional unit,” which can be described as a single-line fracture being spanned by a plate with one screw hole on each side of the fracture. This configuration allows for the simplest determination of worst-case size if the strut geometry is the determining factor for the worst case. If a worst-case size cannot be isolated to a functional unit/strut geometry, perhaps due to irregular screw hole patterns or the shape of the plate, it is understandable that some tests would need to be modified, or possibly removed from test consideration, to accommodate the shape of the plate or the screw hole. Any test modifications or omissions shall be described in the final report with a rationale related to the plate’s anatomical use, indications, and functional requirements.
SCOPE
1.1 This standard is intended to provide guidance for the static testing of small bone metallic plates used for fracture fixation. Small bone plates referred to in this standard would be used in minimally load-bearing anatomical areas of the far extremities, such as the fingers and toes, and in the cranium and upper face. Lower face/mandible, wrist, and ankle fixation plates would generally be larger and carry a substantial amount of load and should not be evaluated under this standard.
1.2 ASTM Specification F382 and ISO 9585 are currently available for the testing of metallic bone plates as well, so the user can choose to use any of the tests in these standards for small bone plates. However, due to plate size, Specification F382 and ISO 9585 test setup and execution difficulty can be increased for small bone plates. Thus, this standard offers alternative test methods that are more appropriate for metallic bone plates used in small bone fracture fixation.
1.3 This standard is not intended to address the mechanical performance of the plating construct or accessory components (for example, screws and wires).
1.4 This standard is intended to provide a basis for the mechanical comparison of small bone plates. Due to the complex and varying biomechanics found in the areas of the body where these plates are used, this standard should only be used to compare the in vitro mechanical performance of small bone plates and not used to infer in vivo performance characteristics.
1.5 This standard describes static tests by specifying load types and specific methods of applying these loads. Tests for evaluating and characterizing these loads include the following: static torsion, static cantilever beam bending, static lateral bending, and static three-point bending.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 Multiple tests are cited in this standard. However, it must be noted that the user is not obligated to test using all of the described methods. Instead, the user should only select test methods that are appropriate for a particular device design.
1.8 This standard does not purport to address all of th...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F3437 − 23
Standard Test Methods for
1
Metallic Bone Plates Used in Small Bone Fracture Fixation
This standard is issued under the fixed designation F3437; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.7 Multiple tests are cited in this standard. However, it
must be noted that the user is not obligated to test using all of
1.1 This standard is intended to provide guidance for the
the described methods. Instead, the user should only select test
static testing of small bone metallic plates used for fracture
methods that are appropriate for a particular device design.
fixation. Small bone plates referred to in this standard would be
1.8 This standard does not purport to address all of the
used in minimally load-bearing anatomical areas of the far
safety concerns, if any, associated with its use. It is the
extremities, such as the fingers and toes, and in the cranium and
responsibility of the user of this standard to establish appro-
upper face. Lower face/mandible, wrist, and ankle fixation
priate safety, health, and environmental practices and deter-
plates would generally be larger and carry a substantial amount
mine the applicability of regulatory limitations prior to use.
of load and should not be evaluated under this standard.
1.9 This international standard was developed in accor-
1.2 ASTM Specification F382 and ISO 9585 are currently
dance with internationally recognized principles on standard-
available for the testing of metallic bone plates as well, so the
ization established in the Decision on Principles for the
user can choose to use any of the tests in these standards for
Development of International Standards, Guides and Recom-
small bone plates. However, due to plate size, Specification
mendations issued by the World Trade Organization Technical
F382 and ISO 9585 test setup and execution difficulty can be
Barriers to Trade (TBT) Committee.
increased for small bone plates. Thus, this standard offers
alternative test methods that are more appropriate for metallic
2. Referenced Documents
bone plates used in small bone fracture fixation.
2
2.1 ASTM Standards:
1.3 This standard is not intended to address the mechanical
E4 Practices for Force Calibration and Verification of Test-
performance of the plating construct or accessory components
ing Machines
(for example, screws and wires).
E2309/E2309M Practices for Verification of Displacement
Measuring Systems and Devices Used in Material Testing
1.4 This standard is intended to provide a basis for the
Machines
mechanical comparison of small bone plates. Due to the
E2624 Practice for Torque Calibration of Testing Machines
complex and varying biomechanics found in the areas of the
F382 Specification and Test Method for Metallic Bone Plates
body where these plates are used, this standard should only be
F983 Practice for Permanent Marking of Orthopaedic Im-
used to compare the in vitro mechanical performance of small
plant Components
bone plates and not used to infer in vivo performance charac-
F2503 Practice for Marking Medical Devices and Other
teristics.
Items for Safety in the Magnetic Resonance Environment
3
1.5 This standard describes static tests by specifying load
2.2 ISO Standards:
types and specific methods of applying these loads. Tests for
ISO 9585 Implants for surgery—Determination of bending
evaluating and characterizing these loads include the follow-
strength and stiffness of bone plates
ing: static torsion, static cantilever beam bending, static lateral
ISO 14602 Non-active surgical implants—Implants for
bending, and static three-point bending.
osteosynthesis—Particular requirements
1.6 The values stated in SI units are to be regarded as
3. Terminology
standard. No other units of measurement are included in this
3.1 Definitions – Geometric:
standard.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
1
These test methods are under the jurisdiction of ASTM Committee F04 on contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Medical and Surgical Materials and Devices and are the direct responsibility of Standards volume information, refer to the standard’s Document Summary page on
Subcommittee F04.21 on Osteosynthesis. the ASTM website.
3
Current edition approved April 1, 2023. Published April 2023. DOI: 10.1520/ Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
F3437-23. 4th Floor, New York,
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.