ASTM D5110-98(2010)
(Practice)Standard Practice for Calibration of Ozone Monitors and Certification of Ozone Transfer Standards Using Ultraviolet Photometry
Standard Practice for Calibration of Ozone Monitors and Certification of Ozone Transfer Standards Using Ultraviolet Photometry
SIGNIFICANCE AND USE
The reactivity and instability of O3 preclude the storage of O3 concentration standards for any practical length of time, and precludes direct certification of O3 concentrations as Standard Reference Materials (SRMs). Moreover, there is no available SRM that can be readily and directly adapted to the generation of O3 standards analogous to permeation devices and standard gas cylinders for sulfur dioxide and nitrogen oxides. Dynamic generation of O3 concentrations is relatively easy with a source of ultraviolet (UV) radiation. However, accurately certifying an O3 concentration as a primary standard requires assay of the concentration by a comprehensively specified analytical procedure, which must be performed every time a standard is needed (9).
This practice is not designed for the routine calibration of O3 monitors at remote locations (see Practices D5011).
SCOPE
1.1 This practice covers a means for calibrating ambient, workplace, or indoor ozone monitors, and for certifying transfer standards to be used for that purpose.
1.2 This practice describes means by which dynamic streams of ozone in air can be designated as primary ozone standards.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. See Section 8 for specific precautionary statements.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D5110 −98 (Reapproved 2010)
Standard Practice for
Calibration of Ozone Monitors and Certification of Ozone
Transfer Standards Using Ultraviolet Photometry
This standard is issued under the fixed designation D5110; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D5011Practices for Calibration of Ozone Monitors Using
Transfer Standards
1.1 This practice covers a means for calibrating ambient,
E220Test Method for Calibration of Thermocouples By
workplace, or indoor ozone monitors, and for certifying
Comparison Techniques
transfer standards to be used for that purpose.
E591Practice for Safety and Health Requirements Relating
1.2 This practice describes means by which dynamic
to Occupational Exposure to Ozone (Withdrawn 1990)
streams of ozone in air can be designated as primary ozone
E644Test Methods for Testing Industrial Resistance Ther-
standards.
mometers
1.3 The values stated in SI units are to be regarded as
3. Terminology
standard. No other units of measurement are included in this
standard. 3.1 Definitions: For definitions of terms used in this
practice, refer to Terminology D1356.
1.4 This standard does not purport to address all of the
3.2 Definitions of Terms Specific to This Standard:
safety concerns, if any, associated with its use. It is the
3.2.1 primary standard—a standard directly defined and
responsibility of the user of this standard to establish appro-
established by some authority, against which all secondary
priate safety and health practices and determine the applica-
standards are compared.
bility of regulatory limitations prior to use. See Section 8 for
specific precautionary statements. 3.2.2 secondary standard—a standard used as a means of
1.5 This international standard was developed in accor- comparison, but checked against a primary standard.
dance with internationally recognized principles on standard-
3.2.3 standard—an accepted reference sample or device
ization established in the Decision on Principles for the
used for establishing measurement of a physical quantity.
Development of International Standards, Guides and Recom-
3.2.4 transfer standard—a type of secondary standard. It is
mendations issued by the World Trade Organization Technical
a transportable device or apparatus that, together with opera-
Barriers to Trade (TBT) Committee.
tional procedures, is capable of reproducing pollutant concen-
tration or producing acceptable assays of pollutant concentra-
2. Referenced Documents
tions.
2.1 ASTM Standards:
3.2.5 zero air—purified air that does not contain ozone, and
D1356Terminology Relating to Sampling and Analysis of
does not contain any other component that may interfere with
Atmospheres
the measurement (see 7.1).
D3195Practice for Rotameter Calibration
D3249Practice for General Ambient Air Analyzer Proce-
4. Summary of Practice
dures
4.1 Thispracticeisbasedonthephotometricassayofozone
D3631Test Methods for Measuring Surface Atmospheric
(O ) concentrations in a dynamic flow system. The concentra-
Pressure
tion of O in an absorption cell is determined from a measure-
ment of the amount of 253.7 nm light absorbed by the sample.
This practice is under the jurisdiction of ASTM Committee D22 on Air
This determination requires knowledge of (1) the absorption
Qualityand is the direct responsibility of Subcommittee D22.03 on Ambient
coefficient of O at 253.7 nm, (2) the optical path length
Atmospheres and Source Emissions.
through the sample, (3) the transmittance of the sample at a
Current edition approved Oct. 1, 2010. Published November 2010. Originally
approved in 1990. Last previous edition approved in 2004 as D5110–98 (2004).
DOI: 10.1520/D5110-98R10.
2 3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or The last approved version of this historical standard is referenced on
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM www.astm.org.
Standards volume information, refer to the standard’s Document Summary page on The boldface numbers in parentheses refer to the references listed at the end of
the ASTM website. this practice.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5110−98 (2010)
wavelength of 253.7 nm, and (4) the temperature and pressure configuration must provide a stable O concentration at the
of the sample. The transmittance is defined as the ratio: systemoutputandallowthephotometertoassayaccuratelythe
output concentration to the precision specified for the photom-
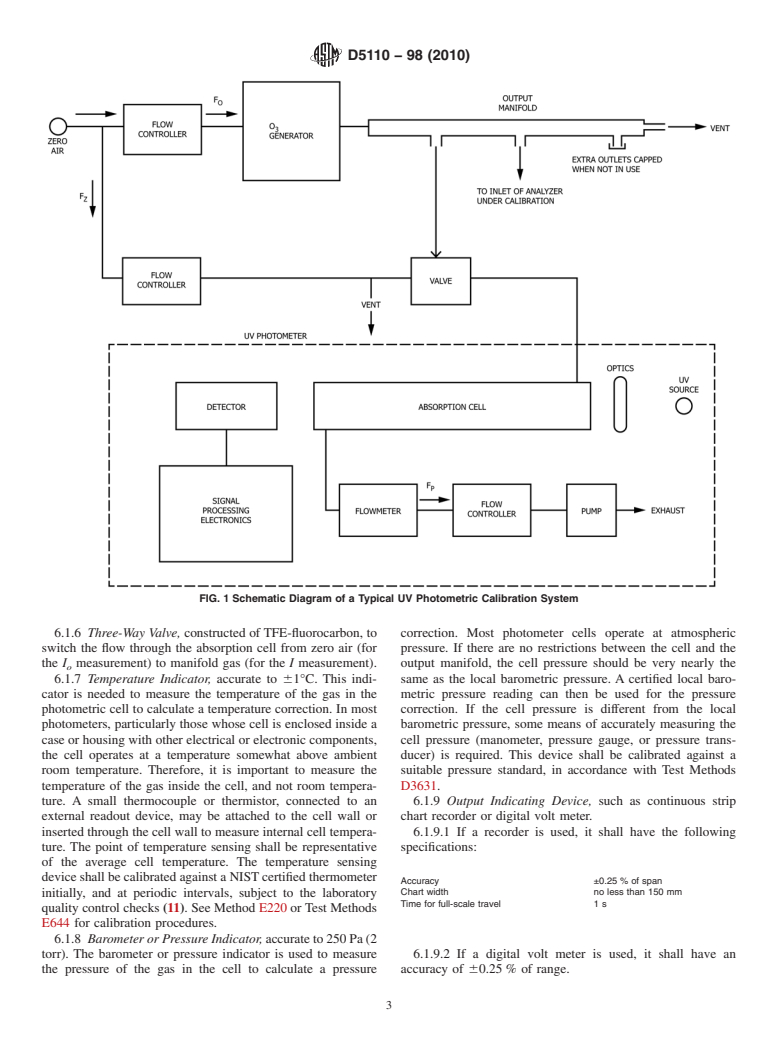
eter. Fig. 1 shows the system, and illustrates the calibration
I/I
o
system. Ozone is highly reactive and subject to losses upon
where:
contact with surfaces.All components between the O genera-
I = the intensity of light that passes through the cell and is tor and the photometer absorption cell shall be of inert
sensed by the detector when the cell contains an O
material, such as glass or TFE-fluorocarbon. Lines and inter-
sample, and connections shall be as short as possible, and all surfaces shall
I = the intensity of light that passes through the cell and is
be chemically clean. For certification of transfer standards that
o
sensed by the detector when the cell contains zero air.
provide their own source of O , the generator and possibly
other components shown in Fig. 1 may not be required (see
Itisassumedthatallconditionsofthesystem,exceptforthe
Practices D5011).
contents of the absorption cell, are identical during measure-
6.1.1 UV Photometer, consisting of a low-pressure mercury
ments of I and I . The quantities defined above are related by
o
the Beer-Lambert absorption law: discharge lamp, collimation optics (optional), an absorption
cell, a detector, and signal-processing electronics, as shown in
Fig. 1. It shall be capable of measuring the transmittance, I/I ,
2acd
o
Transmittance 5 I/I 5 e (1)
o
at a wavelength of 253.7 nm with sufficient precision that the
where:
standarddeviationoftheconcentrationmeasurementsdoesnot
a = absorption coefficient of O at 253.7 nm, exceedthegreaterof0.005ppmor3%oftheconcentration.It
−6 −1 −1
(308 64)×10 ppm cm at 0°C and 101.3 kPa (1 shallincorporatemeanstoassurethatnoO isgeneratedinthe
atm) (1, 2, 3, 4, 5, 6, 7, 8) cell by the UV lamp. This is generally accomplished by
c =O concentration, ppm, and
absorbing the 184.9 nm Hg line with a high silica window, or
d = optical path length, cm.
byisolatingthe253.7nmHglinewithaninterferencefilter.In
addition,atleast99.5%oftheradiationsensedbythedetector
4.1.1 Inpractice,astableO generator(see6.1.4)isusedto
shall be 253.7 nm. This is usually accomplished by using a
produce O concentrations over the required range. Each O
3 3
solar blind photodiode tube. The length of the light path
concentration is determined from the measurement of the
throughtheabsorptioncellshallbeknownwithanaccuracyof
transmittanceofthesampleat253.7nm,andiscalculatedfrom
at least 0.5%. In addition, the cell and associated plumbing
the equation:
shall be designed to minimize loss of O from contact with
surfaces (10).
I
2ln 6.1.2 Air Flow Controller,capableofregulatingairflowsas
I
o
c 5 (2) necessarytomeettheoutputstabilityandphotometerprecision
ad
~ !
requirements.
The calculated O concentrations must be corrected for O
3 3
losses, which may occur in the photometer, and for the tem- 6.1.3 Flowmeters, calibrated in accordance with Practice
perature and pressure of the sample.
D3195.
6.1.4 Ozone Generator, capable of generating stable levels
5. Significance and Use
of O over the required concentration range. It shall be stable
5.1 The reactivity and instability of O preclude the storage
over short periods to facilitate the sequential photometric
of O concentration standards for any practical length of time,
measurement of I and I , and to allow for stability of the
o
and precludes direct certification of O concentrations as
monitor or transfer standard connected to the output manifold.
Standard Reference Materials (SRMs). Moreover, there is no
Conventional UV-photolytic type generators may be adequate,
available SRM that can be readily and directly adapted to the
but shall have line voltage and temperature regulation.
generation of O standards analogous to permeation devices
6.1.5 Output Manifold, constructed of glass, TFE-
and standard gas cylinders for sulfur dioxide and nitrogen
fluorocarbon, or other nonreactive material. It shall be of
oxides. Dynamic generation of O concentrations is relatively
sufficient diameter to ensure a negligible pressure drop at the
easy with a source of ultraviolet (UV) radiation. However,
photometer connection and other output ports. The output
accuratelycertifyinganO concentrationasaprimarystandard
manifoldservesthefunctionofprovidinganinterfacebetween
requires assay of the concentration by a comprehensively
the calibration system and other devices and systems that
specifiedanalyticalprocedure,whichmustbeperformedevery
utilize the output O concentrations. It shall have one or more
time a standard is needed (9).
portsforconnectionoftheexternalinstrumentsorsystems,and
5.2 This practice is not designed for the routine calibration
shallbesuchthatallportsprovidethesameO concentrations.
of O monitors at remote locations (see Practices D5011).
The vent, which exhausts excess gas flow from the system and
insures that the manifold outlet ports are kept at atmospheric
6. Apparatus
pressure for all flowrates, shall be large enough to avoid
6.1 AtypicalcompleteUVcalibrationsystemconsistsofan appreciable pressure drop, and shall be located downstream of
O generator, an output port or manifold, a photometer, a the output ports to ensure that no ambient air enters the
source of zero air, and other components as necessary. The manifold due to eddy currents, back diffusion, and so forth.
D5110−98 (2010)
FIG. 1Schematic Diagram of a Typical UV Photometric Calibration System
6.1.6 Three-Way Valve, constructed ofTFE-fluorocarbon, to correction. Most photometer cells operate at atmospheric
switch the flow through the absorption cell from zero air (for pressure. If there are no restrictions between the cell and the
the I measurement) to manifold gas (for the I measurement). output manifold, the cell pressure should be very nearly the
o
6.1.7 Temperature Indicator, accurate to 61°C. This indi- same as the local barometric pressure. A certified local baro-
cator is needed to measure the temperature of the gas in the metric pressure reading can then be used for the pressure
photometric cell to calculate a temperature correction. In most correction. If the cell pressure is different from the local
photometers, particularly those whose cell is enclosed inside a barometric pressure, some means of accurately measuring the
case or housing with other electrical or electronic components, cell pressure (manometer, pressure gauge, or pressure trans-
the cell operates at a temperature somewhat above ambient ducer) is required. This device shall be calibrated against a
room temperature. Therefore, it is important to measure the suitable pressure standard, in accordance with Test Methods
temperature of the gas inside the cell, and not room tempera- D3631.
ture. A small thermocouple or thermistor, connected to an 6.1.9 Output Indicating Device, such as continuous strip
external readout device, may be attached to the cell wall or chart recorder or digital volt meter.
inserted through the cell wall to measure internal cell tempera- 6.1.9.1 If a recorder is used, it shall have the following
ture. The point of temperature sensing shall be representative specifications:
of the average cell temperature. The temperature sensing
deviceshallbecalibratedagainstaNISTcertifiedthermometer
Accuracy ±0.25 % of span
Chart width no less than 150 mm
initially, and at periodic intervals, subject to the laboratory
Time for full-scale travel 1 s
quality control checks (11). See Method E220 orTest Methods
E644 for calibration procedures.
6.1.8 Barometer or Pressure Indicator,accurateto250Pa(2
torr). The barometer or pressure indicator is used to measure 6.1.9.2 If a digital volt meter is used, it shall have an
the pressure of the gas in the cell to calculate a pressure accuracy of 60.25% of range.
D5110−98 (2010)
7. Reagents and Materials outsideeffects.Ifitisusedforotherpurposes,itwilleventually
become dirty and will be prone to O losses and will give
7.1 Zero Air—Free of O and any substance that by itself or
erraticreadings.Reservingthephotometerforuseasaprimary
whose decomposition products from the ozonizer might react
standard, where only clean, dry, filtered gas passes through the
with O , absorb 255.7 nm light, or undergo photolysis (for
cell, will minimize loss of accuracy. A photometer used as a
example NO, NO , ethylene, and particulate matter). The air
transfer standard will be subjected to environmental
shall be purified to remove such substances. Dirty air shall be
conditions, which may have an effect on its output.
precleaned to remove particulate matter, oil mist, liquid water,
and so forth. 9.3 Photometer Verifications—Since the accuracy of the
7.1.1 The following describes a system that has been used
calibrationstandardsobtainedbythispracticedependsentirely
successfully: The air is dried with a membrane type dryer, ontheaccuracyofthephotometer,itisimportanttoensurethat
followed by a column of indicating silica gel. The air is
the photometer is operating properly and accurately.
irradiated with a UV lamp to generate O , to convert NO to
9.3.1 A well designed and properly built photometer is a
NO andthenpassedthroughacolumnofactivatedcharcoal(6
2 precision instrument; once shown to operate adequately, it is
to 14 mesh) to remove NO,O , hydrocarbons, and various
likely to continue to do so for some time, particularly if it is
2 3
other substances, a column of molecular sieve (6 to 16 mesh,
held stationary and used intermittently under laboratory con-
type 4A), and a final particulate filter (2 µm) to remove
ditions.Therefore,theperformancechecksmaynotnecessarily
particulate matter. (Warning—An important requirement in
have to be conducted every time the photometer is used. The
photometer operation is that the zero air supplied to the
actual frequency of the checks is a trade-off between confi-
photometerduringtheI measurementisfromthesamesource
denceinthephotometerperformanceandthecostandeffortto
o
as that used for
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.