ASTM D4953-06(2012)
(Test Method)Standard Test Method for Vapor Pressure of Gasoline and Gasoline-Oxygenate Blends (Dry Method)
Standard Test Method for Vapor Pressure of Gasoline and Gasoline-Oxygenate Blends (Dry Method)
SIGNIFICANCE AND USE
5.1 Vapor pressure is an important physical property of liquid spark-ignition engine fuels. It provides an indication of how a fuel will perform under different operating conditions. For example, vapor pressure is a factor in determining whether a fuel will cause vapor lock at high ambient temperature or at high altitude, or will provide easy starting at low ambient temperature.
5.2 Petroleum product specifications generally include vapor pressure limits to ensure products of suitable volatility performance. Note 3—Vapor pressure of fuels is regulated by various government agencies.
SCOPE
1.1 This test method covers and is applicable to gasolines and gasoline-oxygenate blends with a vapor pressure range from 35 to 100 kPa (5 to 15 psi) (see Note 2). This test method, a modification of Test Method D323 (Reid Method), provides two procedures to determine the vapor pressure (Note 1) of gasoline and gasoline-oxygenate blends. Note 1—Because the external atmospheric pressure is counteracted by the atmospheric pressure initially present in the air chamber, this vapor pressure is an absolute pressure at 37.8°C (100°F) in kilopascals (pounds-force per square inch). This vapor pressure differs from the true vapor pressure of the sample due to some small vaporization of the sample and air in the confined space.Note 2—Vapor pressure of gasoline or gasoline-oxygenate blends below 35 kPa (5 psi) or greater than 100 kPa (15 psi) can be determined with this test method but the precision and bias (Section 11) do not apply. For materials with a vapor pressure greater than 100 kPa (15 psi), use a 0 to 200 kPa (0 to 30 psi) gauge as specified in the annex of Test Method D323.
1.2 Some gasoline-oxygenate blends may show a haze when cooled to 0 to 1°C. If a haze is observed in 9.4, it shall be indicated in the reporting of results. The precision and bias statements for hazy samples have not been determined (see Note 7).
1.3 The values stated in SI units are to be regarded as standard. The values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific warnings are given in 7.5, 8.4.1, 8.5.1, 9.1, A1.1, and A1.1.3.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D4953 − 06(Reapproved 2012)
Standard Test Method for
Vapor Pressure of Gasoline and Gasoline-Oxygenate Blends
(Dry Method)
This standard is issued under the fixed designation D4953; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method covers and is applicable to gasolines 2.1 ASTM Standards:
and gasoline-oxygenate blends with a vapor pressure range D323 TestMethodforVaporPressureofPetroleumProducts
from35to100kPa(5 to 15 psi) (seeNote 2).This test method, (Reid Method)
D4057 Practice for Manual Sampling of Petroleum and
a modification of Test Method D323 (Reid Method), provides
two procedures to determine the vapor pressure (Note 1)of Petroleum Products
D4175 Terminology Relating to Petroleum, Petroleum
gasoline and gasoline-oxygenate blends.
Products, and Lubricants
NOTE 1—Because the external atmospheric pressure is counteracted by
D5190 Test Method for Vapor Pressure of Petroleum Prod-
the atmospheric pressure initially present in the air chamber, this vapor
ucts (Automatic Method)
pressure is an absolute pressure at 37.8°C (100°F) in kilopascals (pounds-
force per square inch). This vapor pressure differs from the true vapor D5191 Test Method for Vapor Pressure of Petroleum Prod-
pressure of the sample due to some small vaporization of the sample and
ucts (Mini Method)
air in the confined space.
E1 Specification for ASTM Liquid-in-Glass Thermometers
NOTE 2—Vapor pressure of gasoline or gasoline-oxygenate blends
below 35 kPa (5 psi) or greater than 100 kPa (15 psi) can be determined
3. Terminology
with this test method but the precision and bias (Section 11) do not apply.
3.1 Definitions:
For materials with a vapor pressure greater than 100 kPa (15 psi), use a 0
to 200 kPa (0 to 30 psi) gauge as specified in the annex of Test Method
3.1.1 Bourdon spring gauge, n—pressure measuring device
D323.
that employs a bourdon tube connected to an indicator.
1.2 Somegasoline-oxygenateblendsmayshowahazewhen
3.1.2 Bourdon tube, n—flattened metal tube bent to a curve
cooled to 0 to 1°C. If a haze is observed in 9.4, it shall be
that straightens under internal pressure.
indicated in the reporting of results. The precision and bias
3.1.3 dry method, n—in vapor pressure methods, a specific
statements for hazy samples have not been determined (see
empirical test method (D4953) for measuring the vapor pres-
Note 7).
sure of gasoline and other volatile products in which contact of
1.3 The values stated in SI units are to be regarded as
the test specimen with water is not allowed.
standard. The values given in parentheses are for information
3.1.4 dry vapor pressure equivalent (DVPE), n—value cal-
only.
culated by a defined correlation equation, that is expected to be
1.4 This standard does not purport to address all of the
comparable to the vapor pressure value obtained by Test
safety concerns, if any, associated with its use. It is the
Method D4953, Procedure A.
responsibility of the user of this standard to establish appro-
3.1.5 gasoline-oxygenate blend, n—spark-ignition engine
priate safety and health practices and determine the applica-
fuel consisting primarily of gasoline with one or more oxygen-
bility of regulatory limitations prior to use. Specific warnings
ates.
are given in 7.5, 8.4.1, 8.5.1, 9.1, A1.1, and A1.1.3.
3.1.6 oxygenate, n—oxygen-containing ashless organic
compound, such as an alcohol or ether, which may be used as
a fuel or fuel supplement. D4175
This test method is under the jurisdiction of Committee D02 on Petroleum
Products and Lubricants and is the direct responsibility of Subcommittee D02.08 on
Volatility. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Nov. 1, 2012. Published November 2012. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1989. Last previous edition approved in 2006 as D4953–06. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/D4953-06R12. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D4953 − 06 (2012)
3.1.7 vapor pressure, n—pressure exerted by the vapor of a fuels containing oxygenates where the Water Displacement
liquid when in equilibrium with the liquid D4175 Procedure section (10.3.1.8) of D4057 must not be used.
3.2 Abbreviations: 7.4 Sample Container Size:
3.2.1 DVPE, n—dry vapor pressure equivalent 7.4.1 The size of the sample container from which the vapor
pressure sample is taken shall be 1 L (1 qt). It shall be 70 to
4. Summary of Test Method
80 % filled with sample.
7.4.2 The present precision statement has been derived
4.1 The liquid chamber of the vapor pressure apparatus is
using samples in 1-L (1-qt) containers. Samples taken in
filled with the chilled sample and connected to the vapor
containersofothersizesasprescribedinPracticeD4057canbe
chamber at 37.8°C (100°F). The apparatus is immersed in a
used if it is recognized that the precision can be affected. In the
bath at 37.8°C (100°F) until a constant pressure is observed.
case of referee testing the 1-L (1-qt) sample container shall be
The pressure reading, suitably corrected, is reported as the
mandatory.
vapor pressure.
7.5 Hazards:
4.2 Procedure A utilizes the same apparatus and essentially
7.5.1 The vapor pressure determination shall be the first test
the same procedure as Test Method D323 with the exception
withdrawnfromthesamplecontainer.Theremainingsamplein
that the interior surfaces of the liquid and vapor chambers are
the container cannot be used for a second vapor pressure
maintained completely free of water. Procedure B utilizes a
determination. If necessary, obtain a new sample.
semi-automatic apparatus with the liquid and vapor chambers
7.5.2 Samples shall be protected from excessive heat prior
identical in volume to those in Procedure A. The apparatus is
to testing.
suspended in a horizontal bath and rotated while attaining
7.5.3 Samples in leaky containers shall not be tested.
equilibrium.EitheraBourdongaugeorpressuretransducercan
Discard and obtain a new sample.
be used with this procedure. The interior surfaces of the liquid
and vapor chambers are maintained free of water.
7.6 Sample Handling Temperature—In all cases, the sample
container and contents shall be cooled to 0 to 1°C (32 to 34°F)
5. Significance and Use
before the container is opened. Sufficient time to reach this
5.1 Vapor pressure is an important physical property of
temperature shall be assured by direct measurement of the
liquid spark-ignition engine fuels. It provides an indication of
temperature of a similar liquid in a like container placed in the
how a fuel will perform under different operating conditions.
cooling bath at the same time as the sample. See A1.3.1.
For example, vapor pressure is a factor in determining whether
8. Preparation of Apparatus
a fuel will cause vapor lock at high ambient temperature or at
high altitude, or will provide easy starting at low ambient
8.1 This section applies to both ProcedureAand Procedure
temperature. B.
5.2 Petroleum product specifications generally include va-
8.2 Verification of Sample Container Filling—With the
por pressure limits to ensure products of suitable volatility sampleatatemperatureof0to1°C,takethecontainerfromthe
performance.
cooling bath or refrigerator and wipe dry with absorbent
material. If the container is not transparent, unseal it, and using
NOTE 3—Vapor pressure of fuels is regulated by various government
a suitable gauge, confirm that the sample volume equals 70 to
agencies.
80 % of the container capacity (see Note 4). If the sample is
6. Apparatus
contained in a transparent glass container, verify that the
container is 70 to 80 % full by suitable means (see Note 4).
6.1 The apparatus for Procedure A is described in Annex
A1.
NOTE 4—For non-transparent containers, one way to confirm that the
sample volume equals 70 to 80 % of the container capacity is to use a
6.2 Theessentialdimensionsandrequirementsfortheliquid
dipstick that has been pre-marked to indicate the 70 and 80 % container
and vapor chamber for Procedure B are identical with those for
capacities. The dipstick should be of such material that it shows wetting
Procedure A and described in Annex A1. External fittings and
after being immersed and withdrawn from the sample. To confirm the
sample volume, insert the dipstick into the sample container so that it
features will vary depending on whether a gauge or transducer
touches the bottom of the container at a perpendicular angle, before
is used and the provision for rotating the apparatus in the bath.
removing the dipstick. For transparent containers, using a marked ruler or
Details of a commercially available unit are shown in Annex
by comparing the sample container to a like container which has the 70
A2.
and 80 % levels clearly marked, has been found suitable.
8.2.1 Discard the sample if its volume is less than 70 % of
7. Handling of Test Samples
the container capacity.
7.1 This section applies to both Procedure A and B.
8.2.2 If the container is more than 80 % full, pour out
7.2 The extreme sensitivity of vapor pressure measurements enough sample to bring the container contents within 70 to
to losses through evaporation is such as to require the utmost 80 % range. Under no circumstance return any of the poured
precaution and the most meticulous care in handling of out sample to the container.
samples. 8.2.3 Reseal the container, if necessary, and return the
sample container to the cooling bath.
7.3 Sampling shall be done in accordance with the Reid
Vapor Pressure section (10.3) of Practice D4057 except for 8.3 Air Saturation of the Sample in Sample Container:
D4953 − 06 (2012)
8.3.1 Transparent Containers Only—Since 8.2 does not
requirethatthesamplebeopenedtoverifythesamplecapacity,
it is necessary to unseal the cap momentarily before resealing
it, so that the samples in transparent containers are treated the
same as samples in non-transparent containers.
8.3.2 With the sample again at a temperature of 0 to 1°C,
take the container from the cooling bath or refrigerator, wipe it
dry with an absorbent material, remove the cap momentarily,
taking care that no water enters, reseal, and shake vigorously.
Return it to the cooling bath or refrigerator for a minimum of
2 min.
8.3.3 Repeat 8.3.2 twice more. Return the sample to the
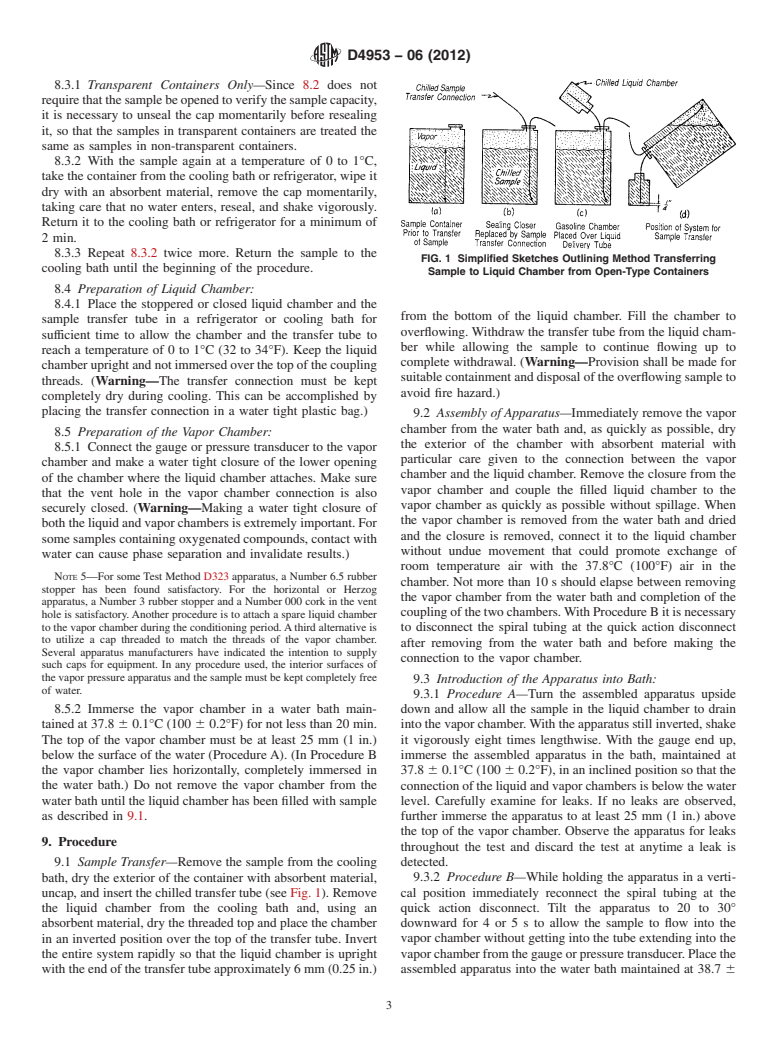
FIG. 1 Simplified Sketches Outlining Method Transferring
cooling bath until the beginning of the procedure.
Sample to Liquid Chamber from Open-Type Containers
8.4 Preparation of Liquid Chamber:
8.4.1 Place the stoppered or closed liquid chamber and the
from the bottom of the liquid chamber. Fill the chamber to
sample transfer tube in a refrigerator or cooling bath for
overflowing. Withdraw the transfer tube from the liquid cham-
sufficient time to allow the chamber and the transfer tube to
ber while allowing the sample to continue flowing up to
reach a temperature of 0 to 1°C (32 to 34°F). Keep the liquid
complete withdrawal. (Warning—Provision shall be made for
chamberuprightandnotimmersedoverthetopofthecoupling
suitable containment and disposal of the overflowing sample to
threads. (Warning—The transfer connection must be kept
avoid fire hazard.)
completely dry during cooling. This can be accomplished by
placing the transfer connection in a water tight plastic bag.)
9.2 Assembly of Apparatus—Immediately remove the vapor
chamber from the water bath and, as quickly as possible, dry
8.5 Preparation of the Vapor Chamber:
the exterior of the chamber with absorbent material with
8.5.1 Connect the gauge or pressure transducer to the vapor
particular care given to the connection between the vapor
chamber and make a water tight closure of the lower opening
chamber and the liquid chamber. Remove the closure from the
of the chamber where the liquid chamber attaches. Make sure
vapor chamber and couple the filled liquid chamber to the
that the vent hole in the vapor chamber connection is also
vapor chamber as quickly as possible without spillage. When
securely closed. (Warning—Making a water tight closure of
the vapor chamber is removed from the water bath and dried
boththeliquidandvaporchambersisextremelyimportant.For
and the closure is removed, connect it to the liquid chamber
some samples containing oxygenated compounds, contact with
without undue movement that could promote exchange of
water can cause phase separation and invalidate results.)
room temperature air with the 37.8°C (100°F) air in the
NOTE 5—For some Test Method D323 apparatus, a Number 6.5 rubber
chamber. Not more than 10 s should elapse between removing
stopper has been found satisfactory. For the horizontal or Herzog
the vapor chamber from the water bath and completion of the
apparatus, a Number 3 rubber stopper and a Number 000 cork in the vent
couplingofthetwochambers.WithProcedureBitisnecessary
hole is satisfactory.Another procedure is to attach a spare liquid chamber
to the vapor chamber during the conditioning period.Athird alternative is
to disconnect the spiral tubing at the quick action disconnect
to utilize a cap threaded to match the threads of the vapor chamber.
after removing from the water bath and before making the
Several apparatus manufacturers have indicated the intention to supply
connection to the vapor chamber.
such caps for equipment. In any procedure used, the interior surfaces of
the vapor pressure apparatus and the sample must be kept completely free
9.3 Introduction of the Apparatus into Bath:
of water.
9.3.1 Procedure A—Turn the assembled apparatus upside
8.5.2 Immerse the vapor chamber in a water bath main- down and allow all the sample in the liquid chamber to drain
tained at 37.8 6 0.1°C (100 6 0.2°F) for not less than 20 min. into the vapor chamber.With the apparatus still inverted, shake
The top of the vapor chamber must be at least 25 mm (1 in.) it vigorously eight times lengthwise. With the gauge end up,
below the surface of the water (ProcedureA). (In Procedure B immerse the assembled apparatus in the bath, maintained at
the vapor chamber lies horizontally, completely immersed in 37.8 6 0.1°C (100 6 0.2°F), in an inclined position so that the
the water bath.) Do not remove the vapor chamber from the connectionoftheliquidandvaporchambersisbelowthewater
water bath until the liquid chamber has been filled with sample level. Carefully examine for leaks.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.