ASTM D4373-96
(Test Method)Standard Test Method for Calcium Carbonate Content of Soils
Standard Test Method for Calcium Carbonate Content of Soils
SCOPE
1.1 This test method covers the quantitative determination of the calcium carbonate (CaCO3) content of soils. It is a gasometric method that utilizes a simple portable apparatus. The test method is quickly performed for soils containing calcium carbonate.
Note 1-The presence of dolomite CaMg(CO3)2 and reducing minerals such as sulfide and sulfate in the soil will interfere with the determination of the amount of CaCO3 present. Therefore, this test method is an approximate method.
1.2 The values stated in SI units are to be regarded as the standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific precaution statements, see Section 6.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
Designation:D4373–96
Standard Test Method for
Calcium Carbonate Content of Soils
This standard is issued under the fixed designation D 4373; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the quantitative determination
of the calcium carbonate (CaCO ) content of soils. It is a
gasometric method that utilizes a simple portable apparatus.
The test method is quickly performed for soils containing
calcium carbonate.
NOTE 1—The presence of dolomite CaMg(CO ) and reducing miner-
3 2
als such as sulfide and sulfate in the soil will interfere with the
determination of the amount of CaCO present. Therefore, this test
method is an approximate method that determines the calcium carbonate
equivalent.
1.2 The values stated in SI units are to be regarded as the
standard.
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. For specific
precaution statements, see Section 6.
2. Summary of Test Method
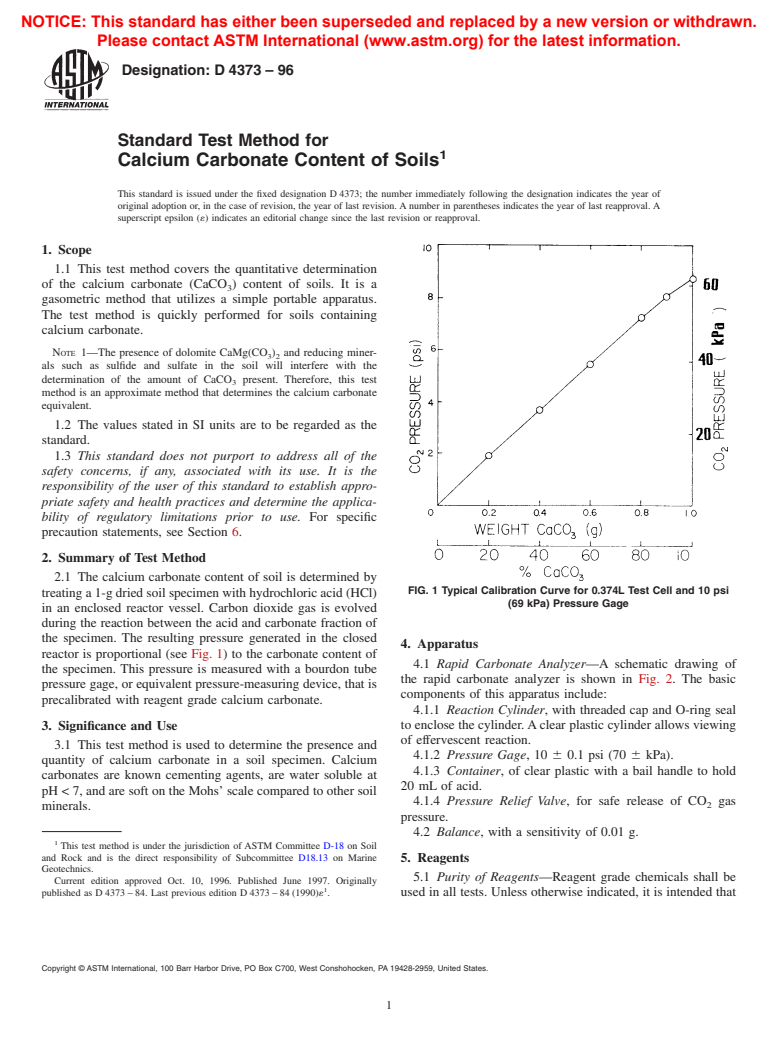
2.1 The calcium carbonate content of soil is determined by
FIG. 1 Typical Calibration Curve for 0.374L Test Cell and 10 psi
treating a 1-g dried soil specimen with hydrochloric acid (HCl)
(69 kPa) Pressure Gage
in an enclosed reactor vessel. Carbon dioxide gas is evolved
during the reaction between the acid and carbonate fraction of
the specimen. The resulting pressure generated in the closed
4. Apparatus
reactor is proportional (see Fig. 1) to the carbonate content of
4.1 Rapid Carbonate Analyzer—A schematic drawing of
the specimen. This pressure is measured with a bourdon tube
the rapid carbonate analyzer is shown in Fig. 2. The basic
pressure gage, or equivalent pressure-measuring device, that is
components of this apparatus include:
precalibrated with reagent grade calcium carbonate.
4.1.1 Reaction Cylinder, with threaded cap and O-ring seal
3. Significance and Use to enclose the cylinder.Aclear plastic cylinder allows viewing
of effervescent reaction.
3.1 This test method is used to determine the presence and
4.1.2 Pressure Gage,10 6 0.1 psi (70 6 kPa).
quantity of calcium carbonate in a soil specimen. Calcium
4.1.3 Container, of clear plastic with a bail handle to hold
carbonates are known cementing agents, are water soluble at
20 mL of acid.
pH < 7, and are soft on the Mohs’ scale compared to other soil
4.1.4 Pressure Relief Valve, for safe release of CO gas
minerals.
pressure.
4.2 Balance, with a sensitivity of 0.01 g.
This test method is under the jurisdiction of ASTM Committee D-18 on Soil
and Rock and is the direct responsibility of Subcommittee D18.13 on Marine
5. Reagents
Geotechnics.
5.1 Purity of Reagents—Reagent grade chemicals shall be
Current edition approved Oct. 10, 1996. Published June 1997. Originally
published as D 4373 – 84. Last previous edition D 4373 – 84 (1990)´ . used in all tests. Unless otherwise indicated, it is intended that
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
D4373–96
FIG. 2 Schematic Drawing of Rapid Carbonate Analyzer
all reagents shall conform to the specifications of the Commit- 6.2 The pressure relief valve of the carbonate analyzer
tee onAnalytical Reagents of theAmerican Chemical Society, should be opened following each test to dissipate gas pressure
where such specifications are available. Other grades may be
so that the cap can be safely removed.
used, provided it is first ascertained that the reagent is of
sufficiently high purity to permit its use without lessening the
7. Test Specimens
accuracy of the determination.
7.1 Select 20 or 30-g specimens from a core or surface grab
5.2 Calcium Carbonate (CaCO ).
sample.Ovendryat105°Cforaperiodof12to24h.Pulverize
5.3 Hydrochloric Acid (HCl), (in about 1 N solution)—
theentiresamplewithamortarandpestle(orhammer)untilall
Prepare 1 L of about 1 N solution by placing 80 mL of
of the particles pass a No. 10 (2-mm) sieve. Smaller particles
concentrated, reagent grade HCl in a 1L volumetric flask and
react faster than larger particles when treated with acid.
dilute to the mark with distilled or demineralized water. Store
in polyethylene bottle.
8. Calibration
6. Safety Precautions
8.1 Calibration is accomplished by using reagent grade
6.1 Use care in handling the hydrochloric acid so that no
CaCO toobtaintherelationshipbetweentheweightofCaCO
3 3
acid is spil
...






Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.