ASTM G96-90(2001)e1
(Guide)Standard Guide for On-Line Monitoring of Corrosion in Plant Equipment (Electrical and Electrochemical Methods)
Standard Guide for On-Line Monitoring of Corrosion in Plant Equipment (Electrical and Electrochemical Methods)
SIGNIFICANCE AND USE
General corrosion is characterized by areas of greater or lesser attack, throughout the plant, at a particular location, or even on a particular probe. Therefore, the estimation of corrosion rate as with mass loss coupons involves an averaging across the surface of the probe. Allowance must be made for the fact that areas of greater or lesser penetration usually exist on the surface. Visual inspection of the probe element, coupon, or electrode is required to determine the degree of interference in the measurement caused by such variability. This variability is less critical where relative changes in corrosion rate are to be detected.
Both electrical test methods described in this guide provide a technique for determining corrosion rates without the need to physically enter the system to withdraw coupons as required by the methods described in Guide G 4.
Test Method B has the additional advantage of providing corrosion rate measurement within minutes.
These techniques are useful in systems where process upsets or other problems can create corrosive conditions. An early warning of corrosive attack can permit remedial action before significant damage occurs to process equipment.
These techniques are also useful where inhibitor additions are used to control the corrosion of equipment. The indication of an increasing corrosion rate can be used to signal the need for additional inhibitor.
Control of corrosion in process equipment requires a knowledge of the rate of attack on an ongoing basis. These test methods can be used to provide such information in digital format easily transferred to computers for analysis. TEST METHOD A—ELECTRICAL RESISTANCE
(1-6)4 Top
SCOPE
1.1 This guide outlines the procedure for conducting on-line corrosion monitoring of metals in plant equipment under operating conditions by the use of electrical or electrochemical methods. Within the limitations described, these test methods can be used to determine cumulative metal loss or instantaneous corrosion rate, intermittently or on a continuous basis, without removal of the monitoring probes from the plant.
1.2 The following test methods are included: Test Method A for electrical resistance, and Test Method B for polarization resistance.
1.2.1 Test Method A provides information on cumulative metal loss, and corrosion rate is inferred. This test method responds to the remaining metal thickness except as described in Section 5.
1.2.2 Method B is based on electrochemical measurements for determination of instantaneous corrosion rate but may require calibration with other techniques to obtain true corrosion rates. Its primary value is the rapid detection of changes in the corrosion rate that may be indicative of undesirable changes in the process environment.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in 5.6.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
e1
Designation:G96–90(Reapproved 2001)
Standard Guide for
On-Line Monitoring of Corrosion in Plant Equipment
(Electrical and Electrochemical Methods)
ThisstandardisissuedunderthefixeddesignationG96;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—The Appendix section was editorially corrected in October 2001.
1. Scope rosion Test Specimens
G3 PracticeforConventionsApplicabletoElectrochemical
1.1 Thisguideoutlinestheprocedureforconductingon-line
Measurements in Corrosion Testing
corrosion monitoring of metals in plant equipment under
G4 Guide for Conducting Corrosion Tests in Field Appli-
operatingconditionsbytheuseofelectricalorelectrochemical
cations
methods. Within the limitations described, these test methods
G15 Terminology Relating to Corrosion and Corrosion
can be used to determine cumulative metal loss or instanta-
Testing
neous corrosion rate, intermittently or on a continuous basis,
G59 Test Method for Conducting Potentiodynamic Polar-
without removal of the monitoring probes from the plant.
ization Resistance Measurements
1.2 Thefollowingtestmethodsareincluded:TestMethodA
G102 Practice for Calculation of Corrosion Rates and
for electrical resistance, and Test Method B for polarization
RelatedInformationfromElectrochemicalMeasurements
resistance.
1.2.1 Test Method A provides information on cumulative
3. Terminology
metal loss, and corrosion rate is inferred. This test method
3.1 Definitions—See Terminology G15 for definitions of
responds to the remaining metal thickness except as described
terms used in this guide.
in Section 5.
1.2.2 Method B is based on electrochemical measurements
4. Summary of Guide
for determination of instantaneous corrosion rate but may
4.1 Test Method A–Electrical Resistance—The electrical
require calibration with other techniques to obtain true corro-
resistance test method operates on the principle that the
sionrates.Itsprimaryvalueistherapiddetectionofchangesin
electricalresistanceofameasuringelement(wire,strip,ortube
the corrosion rate that may be indicative of undesirable
of metal) increases as its cross-sectional area decreases:
changes in the process environment.
l
1.3 This standard does not purport to address all of the
R5s (1)
A
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
where:
priate safety and health practices and determine the applica-
R = resistance,
bility of regulatory limitations prior to use. Specific precau-
s = resistivity of metal (temperature dependent),
tionary statements are given in 5.6.
l = length, and
A = cross-section area.
2. Referenced Documents
In practice, the resistance ratio between the measuring
2.1 ASTM Standards:
element exposed to corrosion and the resistance of a similar
D1125 Test Methods for Electrical Conductivity and Re-
reference element protected from corrosion is measured, to
sistivity of Water
compensate for resistivity changes due to temperature. Based
G1 Practice for Preparing, Cleaning, and Evaluating Cor-
on the initial cross-sectional area of the measurement element,
the cumulative metal loss at the time of reading is determined.
Metal loss measurements are taken periodically and manually
This guide is under the jurisdiction ofASTM Committee G01 on Corrosion of
or automatically recorded against a time base.The slope of the
Metals and is the direct responsibility of ASTM Subcommittee G01.11 on
Electrochemical Measurements in Corrosion Testing.
Current edition approved March 30, 1990. Published May 1990.
2 3
Annual Book of ASTM Standards, Vol 11.01. Annual Book of ASTM Standards, Vol 03.02.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
e1
G96–90 (2001)
curve of metal loss against time at any point is the correction
where:
rateatthatpoint.Themorefrequentlymeasurementsaretaken,
K = a constant.
the better is the resolution of the curve from which the
4.2.4 Equivalent weight of an element is the molecular
corrosion rate is derived.
weight divided by the valency of the reaction (that is, the
4.1.1 The electrical resistance of the metal elements being
number of electrons involved in the electrochemical reaction).
measured is very low (typically 2 to 10 mV). Consequently,
4.2.5 In order to obtain an alloy equivalent weight that is in
special measurement techniques and cables are required to
proportion with the mass fraction of the elements present and
minimize the effect of cable resistance and electrical noise.
their valence, it must be assumed that the oxidation process is
4.1.2 Various probe element cross-sectional areas are nec-
uniformanddoesnotoccurselectively;thatis,eachelementof
essarysothatawiderangeofcorrosionratescanbemonitored
the alloy corrodes as it would if it were the only element
with acceptable resolution.
present. In some situations these assumptions are not valid.
4.2 Test Method B–Polarization Resistance:
4.2.6 Effective equivalent weight of an alloy is as follows:
4.2.1 Thepolarizationresistancetestmethodinvolvesinter-
actionwiththeelectrochemicalcorrosionmechanismofmetals
(5)
m
nf
i i
in electrolytes in order to measure the instantaneous corrosion
(
W
l
i
rate. Its particular advantage is its speed of response to
corrosion rate upsets. On a corroding electrode subject to
where:
certain qualifications (see 12.1), it has been shown that the
f = mass fraction of i element in the alloy,
i th
current density associated with a small polarization of the
W = atomic weight of the i element in the alloy,
i th
electrode is directly proportional to the corrosion rate of the
n = exhibited valence of the i element under the condi-
i th
electrode.
tions of the corrosion process, and
4.2.2 The polarization resistance equation is derived in Test
m = numberofcomponentelementsinthealloy(normally
MethodG59.SeePracticeG3forapplicableconventions.For
only elements above 1 mass% in the alloy are
smallpolarizationoftheelectrode(typically DEupto20mV),
considered).
the corrosion current density is defined as:
Alloy equivalent weights have been calculated for many
B engineering metals and alloys and are tabulated in Practice
i 5 (2)
corr
R
G102.
p
4.2.7 Fig. 1 represents an equivalent circuit of polarization
where:
resistance probe electrodes in a corroding environment. The
B = acombinationoftheanodicandcathodicTafelslopes
value of the double layer capacitance, C , determines the
dl
( b,b ), and
a c
2 charging time before the current density reaches a constant
R = the polarization resistance with dimensions ohm·cm .
p
value, i, when a small potential is applied between the test and
auxiliary electrode. In practice, this can vary from a few
b b
a c
seconds up to hours. When determining the polarization
B 5 (3)
2.303 ~b 1 b !
a c
resistance, R , correction or compensation for solution resis-
p
4.2.3 The corrosion current density, i , can be converted
tance, R , is important when R becomes significant compared
corr
s s
to corrosion rate of the electrode by Faraday’s law if the
to R . Test Methods D1125 describes test methods for electri-
p
equivalent weight (EW) and density, r, of the corroding metal
cal conductivity and resistivity of water.
are known (see Practice G102):
4.2.8 Two-electrodeprobes,andthree-electrodeprobeswith
the reference electrode equidistant from the test and auxiliary
i
corr
corrosionrate 5 K EW (4)
r electrode, do not correct for effects of solution resistance,
−2
NOTE 1—R =Solution Resistance (ohm·cm ) between test and auxiliary electrodes (increases with electrode spacing and solution resistivity).
s
−2
R =Uncompensated component of solution resistance (between test and reference electrodes) (ohm·cm ).
u
R =Polarization Resistance R (ohm·cm ).
p p
Cdl=Double layer capacitance of liquid/metal interface.
i=Corrosion current density.
FIG. 1 Equivalent Circuit of Polarization Resistance Probe
e1
G96–90 (2001)
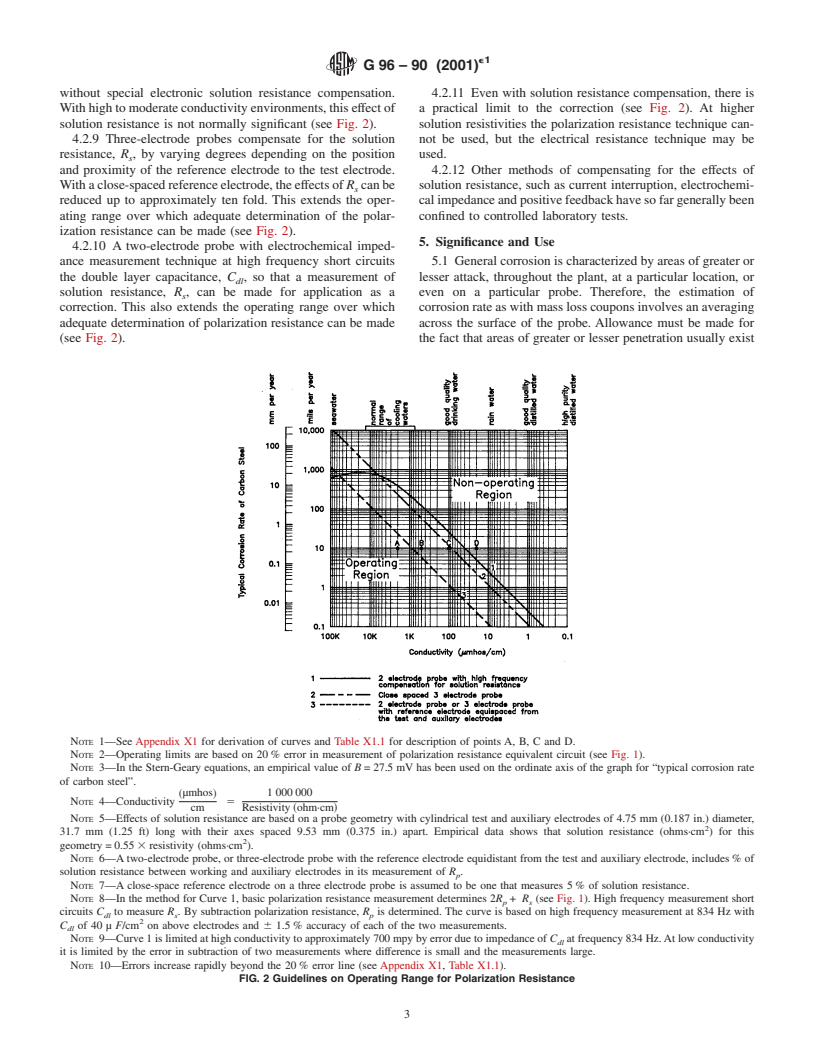
without special electronic solution resistance compensation. 4.2.11 Even with solution resistance compensation, there is
Withhightomoderateconductivityenvironments,thiseffectof a practical limit to the correction (see Fig. 2). At higher
solution resistance is not normally significant (see Fig. 2). solution resistivities the polarization resistance technique can-
4.2.9 Three-electrode probes compensate for the solution not be used, but the electrical resistance technique may be
resistance, R , by varying degrees depending on the position used.
s
and proximity of the reference electrode to the test electrode. 4.2.12 Other methods of compensating for the effects of
Withaclose-spacedreferenceelectrode,theeffectsofR canbe solution resistance, such as current interruption, electrochemi-
s
reduced up to approximately ten fold. This extends the oper- calimpedanceandpositivefeedbackhavesofargenerallybeen
ating range over which adequate determination of the polar- confined to controlled laboratory tests.
ization resistance can be made (see Fig. 2).
5. Significance and Use
4.2.10 A two-electrode probe with electrochemical imped-
ance measurement technique at high frequency short circuits 5.1 Generalcorrosionischaracterizedbyareasofgreateror
the double layer capacitance, C , so that a measurement of lesser attack, throughout the plant, at a particular location, or
dl
solution resistance, R , can be made for application as a even on a particular probe. Therefore, the estimation of
s
correction. This also extends the operating range over which corrosionrateaswithmasslosscouponsinvolvesanaveraging
adequate determination of polarization resistance can be made across the surface of the probe. Allowance must be made for
(see Fig. 2). the fact that areas of greater or lesser penetration usually exist
NOTE 1—See Appendix X1 for derivation of curves and Table X1.1 for description of points A, B, C and D.
NOTE 2—Operating limits are based on 20% error in measurement of polarization resistance equivalent circuit (see Fig. 1).
NOTE 3—In the Stern-Geary equations, an empirical value of B=27.5 mV has been used on the ordinate axis of the graph for “typical corrosion rate
of carbon steel”.
~µmhos! 1000000
NOTE 4—Conductivity 5
cm Resistivity ~ohm·cm!
NOTE 5—Effects of solution resistance are based on a probe geometry with cylindrical test and auxiliary electrodes of 4.75 mm (0.187 in.) diameter,
31.7 mm (1.25 ft) long with their axes spaced 9.53 mm (0.375 in.) apart. Empirical data shows that solution resistance (ohms·cm ) for this
geometry=0.55 3resistivity (ohms·cm ).
NOTE 6—Atwo-electrode probe, or three-electrode probe with the reference electrode equidistant from the test and auxiliary electrode, includes% of
solution resistance between working and auxiliary electrodes in its measurement of R .
p
NOTE 7—A close-space reference electrode on a three electrode probe is assumed to be one that measures 5% of solution resistance.
NOTE 8—In the method for Curve 1, basic polarization resistance measurement determines 2R + R (see Fig. 1). High frequency measurement short
p s
circuits C to measure R . By subtraction polarization resistance, R is determined. The curve is based on high frequency measurement at 834 Hz with
dl s p
C of 40 µ F/cm on above electrodes and 6 1.5% accuracy of each of the two measurements.
dl
NOTE 9—Curve1islimitedathighconductivitytoapproximately700mpybyerrorduetoimpedanceof C atfrequency834Hz.Atlowconductivity
dl
it is limited by the error in subtraction of two measurements where difference is small and the measurements large.
NOTE 10—Errors increase rapidly beyond the 20% error line (see Appendix X1, Table X1.1).
FIG. 2 Guidelines on Operating Range for Polarization Resistance
e1
G96–90 (2001)
onthesurface.Visualinspectionoftheprobeelement,coupon, 6.2.2 If probe elements are flexed due to excessive flow
or electrode is required to determine the degree of interference conditions, a strain gage effect can be produced introducing
in the measurement caused by such variability.This variability stress noise onto the probe measurement. Suitable probe
islesscriticalwhererelativechangesincorrosionratearetobe element shielding can remove such effects.
detected. 6.3 Process fluids, except liquid metals and certain molten
5.2 Both electrical test methods described in this guide salts, do not normally have sufficient electrical conductivity to
provideatechniquefordeterminingcorrosionrateswithoutthe produceasignificantshortingeffectontheelectricalresistance
need to physically enter the system to withdraw coupons as of the exposed probe element. Conductive deposits (such as
required by the methods described in GuideG4. iron sulphide) can cause some short circuiting effect on the
5.3 Test Method B has the additional advantage of provid- element, reducing the measured metal loss, or showing some
ing corrosion rate measurement within minutes. apparent metal gain. Certain probe configurations are less
5.4 These techniques are useful in systems where process sensitive to this than others, depending on the path length
upsets or other problems can create corrosive conditions. An between one end of the exposed probe element and the other.
early warning of corrosive attack can permit remedial action 6.4 When first introduced into a system, initial transient
before significant damage occurs to process equipment. corrosion rates on a probe element may be different from the
5.5 These techniques are also useful where inhibitor addi- longer term corrosion rates.
tions are used to control the corrosion of equipment. The 6.4.1 Establishment of a probe element surface typical of
indication of an increasing corrosion rate can be used to signal the plant by passivation, oxidation, deposits, or inhibitor film
the need for additional inhibitor. build up may vary from hours to several days.
5.6 Control of corrosion in process equipment requires a 6.5 Since the corrosion rate is usually temperature depen-
knowledgeoftherateofattackonanongoingbasis.Thesetest dent, results will be comparable only for the alloy at the
methods can be used to provide such information in digital process temperature to which the probes are exposed. In heat
format easily transferred to computers for analysis. transfer environments actual plant metal temperatures may be
significantly different from that of the test probe.
TEST METHOD A—ELECTRICAL RESISTANCE 6.6 Electrical resistance probe elements are by their nature
consumable. Hazardous situations may occur i
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.